AP Chemistry Collision Theory/Transition State Theory
advertisement

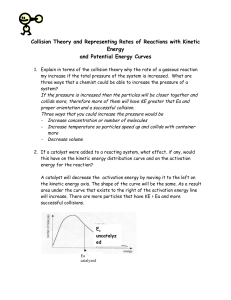
AP Chemistry Collision Theory/Transition State Theory Both Collision Theory and Transition State Theory work in conjunction to explain observed kinetic phenomenon. They both utilize the idea of an Activation Energy (Ea) to explain data, but approach it from a slightly different perspective. Collision Theory based on kinetic theory and assumes a collision between reactants before a reaction can take place. postulates that the majority of collisions do not lead to a reaction, but only those in which the colliding species have: o a kinetic energy greater than a certain minimum, called the activation energy, Ea o the correct spatial orientation with respect to each other can be used to explain… o the effect of concentration on rate o the effect of temperature on rate (see below – Arrhenius discussion, activation energy) If either or both of the above two pre-requisites, correct reaction geometry and sufficient kinetic energy, are not met, then the collision does not lead to the formation of products and the reactants simply "bounce" off each other. However as the temperature increases, not only does the number of collisions per second increase (and hence the number of collisions in the correct orientation per second) but also the proportion of collisions that have a kinetic energy equal to or greater than Ea, the activation energy. The combination of these two effects, and particularly the second, is used to explain the marked effect of tempearture on reaction rate in the collsion theory. Arrhenius – Colliding particles must have an energy > a threshold energy called the Activation Energy, Ea (see below graph). As the temperature increases, the fraction of collisions with E > Ea increases (see above graph). Mathematically, the rate constant k is explained as. . . k = Ae-Ea/RT where A = frequency factor = product of collision frequency z and an orientation probability factor p A = pz think of p as a percentage of effective collisions; p = # effective collisions/total # collisions Transition State Theory describes an activated complex, or transition state, of a reaction envisions the kinetic energy of the particles changing to potential energy during a collision the activated complex forms only if the energy > Ea and the orientation of collision is correct the activation energy is used to stretch and deform bonds in order to reach the transition state proposes that every reaction goes through its own transition state, from which the reaction can go in either direction (all reactions are reversible) In the figures below, notice a few things… 1. For every reaction there exists a forward (Ea,f) and reverse activation energy (Ea,r), with the values rarely ever equal. 2. The difference between the two activation energies is the heat of the reaction (Hrxn) 3. The size of the Ea will usually give information on which direction the reaction will favor (forward or reverse)