Molecular Motion in Gases
advertisement

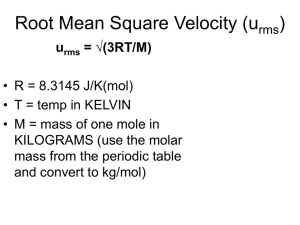
Chapter 21: Molecules in motion • Diffusion: the migration of matter down a concentration gradient. • Thermal conduction: the migration of energy down a temperature gradient. • Electric conduction: the migration of electric charge along an electrical potential gradient. • Viscosity: the migration of linear momentum down a velocity gradient. • Effusion: the emergence of a gas from a container through a small hole. 21.1 The kinetic model of gases • Three assumptions: 1. The gas consists of molecules of mass m in ceaseless random motion. 2. The size of the molecules is negligible, in the sense that their diameters are much smaller than the average distance traveled between collisions. 3. The molecules interact only through brief, infrequent, and elastic collisions. (Elastic collision: a collision in which the total translational kinetic energy of the molecules is conserved.) Pressure and Molecular speeds PV = 1/3 nMc2 (21.1) where M = mNA, the molar mass of the molecules, c is the root mean square speed of the molecules: c2 = vx2 + vy2 + vz2 c = < vx2 + vy2 + vz2 >1/2 (21.2) Relationship between temperature and the root mean square speed • Provided that the root mean square speed of the molecules depends only on the temperature: pV = constant at constant temperature • In comparison with Boyle’s law, one gets 1/ 2 3 RT c= (21.3) M • The root mean square speed of the gas molecules is proportional to the square root of temperature and inversely proportional to the square root of the molar mass. Maxwell distribution of speeds M f (v) 4 2RT 3/ 2 2 Mv 2 / 2 RT v e (21.4) Fraction in the range v1 to v2 equals v2 v 1 f (v )dv Expression of molecular speeds • Mean speed • The most probable speed • 1/ 2 8RT c M 2 RT c* M 1/ 2 Relative mean speed: 1/ 2 8kT c rel 21 / 2 c m A mB m A mB (reduced mass) _ _ Measuring molecular speed The collision frequency • Collision diameter: the actual diameter of the molecule. • Collision frequency (z): the number of collisions made by one molecule divided by the time interval during which the collisions are counted. • Collision cross-section: σ = πd2 _ • Z (c rel t ) N t crel P kT N is number density = Mean free path • Mean free path, λ, the average distance a molecule travels between collisions. _ c z kT 21 / 2 P • How does temperature or pressure affect the mean free path ? • The distance between collisions is determined by the number of molecules present in the given volume, not by the speed at which they travel. 21.2 Collisions with walls and surfaces • The collision flux, Zw, the number of collisions with the area in a given time interval divided by the area and the duration of the interval. p 1 Zw cN 1/ 2 (2m kT) 4 • Collision frequency can be obtained by multiplication of the collision flux by the area of interest. 21.3 The rate of effusion • Graham’s law of effusion: the rate of effusion is inversely proportional to the square root of the molar mass. rate of effusion Z w A0 pA0 (2mkT)1 / 2 pA0 N A (2MRT )1 / 2 • Vapor pressures of liquids and solids can be measured based on the above equation. ( Knudsen method) Example 21.2 Caesium (m.p. 29oC, b.p. 686 oC) was introduced into a container and heated to 500 oC. When a hole of diameter 0.500mm was opened in the container for 100s, a mass loss of 385 mg was measured. Calculate the vapor pressure of liquid cesium at 500K. Solution: Despite the effusion, the vapor pressure is constant inside the container because the hot liquid metal replenishes the vapor. Consequently, the effusion rate is constant! The mass loss Δm in an interval Δt is related to the collision flux by: Δm = (ZwA0) Δt m Where A0 is the area of the hole and m is the mass of one Caesium atom. ZW m A0 mt p (2mkT)1 / 2 m A0mt plug in numbers, one gets p = 11k Pa • Self-test 21.2: How long would it take 1.0 g of Cs atoms to effuse out of the over under the same conditions as listed in example 21.2? (260 s) • Self-test: There is 1.0 g of Cs solid in the effusion oven, how long does it take to effuse out of the oven?