If molecules were people
advertisement

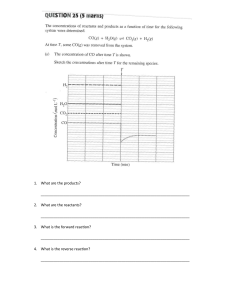
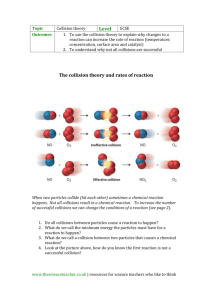
Name _____________________________________ Period ____ If molecules were people... http://ed.ted.com/lessons/if-molecules-were-people-george-zaidan-and-charles-morton 1. What is/are the requirement(s) for a collision to result in a reaction? a. Both proper orientation and enough energy b. Only proper orientation c. Only enough energy d. Either proper orientation or enough energy 416845 2. Which of the following statements is true: a. All chemical reactions happen in one direction (forward) 416846 416846 b. All chemical reactions happen in both directions (forward and reverse) c. Some chemical reactions happen only in the forward direction, but most happen in both 416846 directions d. Some chemical reactions happen only in the forward direction, and some happen only in the reverse direction 3. At the very beginning of a reversible reaction, what is the rate of the reverse reaction? a. Slower than the forward reaction, but greater than zero b. Zero 416847 416847 c. Faster than the forward reaction d. Impossible to tell 416847 4. What is the ratio between reactants and products after equilibrium has been established? a. It’s always 1:1 (50/50) 416848 b. It could be 1:1, but it could also be anything else 416848 c. It’s never 1:1 d. It’s always 1:2 416848 5. How do chemists measure the rate of reactions and the position of equilibria? a. By doing a bunch of experiments in the lab 416849 b. By looking closely at the molecular structures of the reactants and products 416849 416849 c. By looking up the values in books d. By typing the reaction into a computer program 6. Look at the first four collisions in the video. In collision A, two people bump into each other. In collision B, two molecules (the large one is ethene, the small one is hydrogen) bump into each other. In collision C, the same two people bump into each other; and in collision D, the same two molecules bump into each other. In collisions A and B, no reaction happens. In collisions C and D, reactions do happen. Look closely at the four collisions and give two reasons why reactions happen in cases C and D but not in cases A and B. 7. Explain why the rate of the reverse reaction must be zero at the very, very beginning of a reversible reaction. Explain why the rate of the reverse reaction increases as the reaction proceeds. 8. Explain why the proportion of reactants and products at equilibrium does not have to be 50/50.