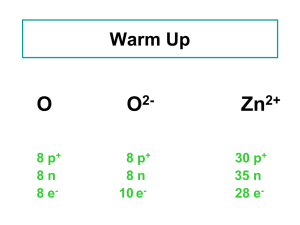
Isotopes are atoms of the same element that have a different number
advertisement
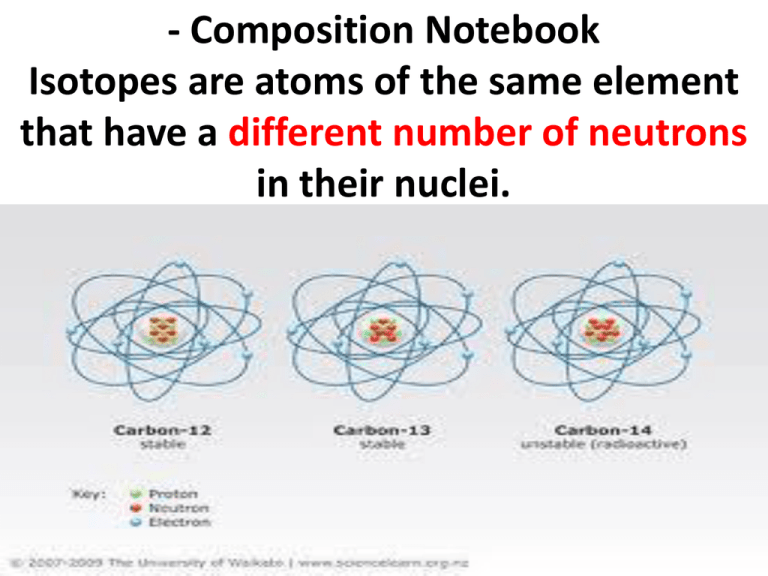
- Composition Notebook Isotopes are atoms of the same element that have a different number of neutrons in their nuclei. Protons make the element what it is ! • Isotopes of an element will have the same number of protons but a different number of neutrons. • The number of protons in the nucleus of an atom and is the same for all atoms of the same element. Mass Number •is the number of protons + the number of neutrons in the nucleus of an atom and will be different for each isotope of an element. Isotope classification • Isotopes can be classified as natural (found in nature) or man made (artificial or synthetic). • Isotopes can also be classified as stable or unstable Stable vs. Unstable • A stable isotope does NOT undergo radioactive (or nuclear) decay. • An unstable isotope undergoes radioactive (or nuclear) decay. Unstable isotopes are also known as radioisotopes or radionucleides Examples of Isotopes- atoms with different numbers of neutronsnaturally occurring isotopes • • • • • • • • Hydrogen- 1 and Hydrogen -2 Helium-3 and Helium-4 (one extra neutron) Lithium-3 and Lithium-7 (4 extra neutrons) Boron-10 and Boron-11 (one extra neutron) Carbon-12, Carbon-13, Carbon-14 Nitrogen-14 and Nitrogen-15 Oxygen-16, Oxygen-17, and Oxygen-18 Neon- 20, Neon-21, and Neon-22 How are the carbon atoms different? Unstable Isotopes • Carbon-14 (two more neutrons than C-12) • Uranium-234, Uranium-235, Uranium-238 Practice Drawing Isotopes Helium-3 and Helium-4 (one extra neutron) Lithium-3 and Lithium-7 (4 extra n Practice Drawing Isotopes Lithium-3 and Lithium-7 (4 extra neutrons) Boron-10 and Boron-11 (one extra neutron) Carbon-12, Carbon-13, Carbon-14 Radioactivity • A nucleus of an atom is unstable and releases subatomic particles. • Radioactivity is a random process, meaning that it is physically impossible to predict whether or not a given atomic nucleus will decay and emit radiation at any given moment United Streaming Resources Radioactivity: Nuclear Disintegration and subatomic particles Radium and Radioactivity 2 min clip Welcome to Discovery Education Player Follow Up Questions • What is an isotope? • What subatomic particle makes an atom an isotope? • Do isotopes have different numbers of protons? Follow Up Questions • What is radioactivity? • Where does radioactivity happen in the atom? • What is released when an atom decays? • What happens to the atom when it decays? Does it change?