Nuclear Chemistry: Islands of Stability Lesson
advertisement

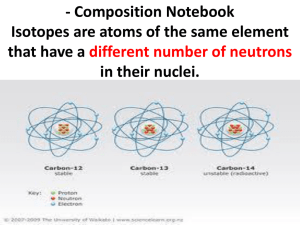
Nuclear Chemistry Lesson 1: Islands of Stability Nuclear Chemistry – Bell work The isotope notation for an atom of copper and an atom of gold are given below: 63 197 29 79 Cu Au • How could you change a copper atom into a gold atom? • What would you need to change? Give specific numbers. • Why is this change called a nuclear reaction? The Big Question • What is the range of the number of neutrons found in isotopes of various elements? • Goal - Determine how many neutrons are required to make a stable element with a given number of protons. Island of stability Plot this isotope 41 19 K # of protons? # of neutrons? protons = neutrons Band of stability Notes • Nuclear chemistry study of atom nucleus • Band of stability range in neutron # for given proton # for naturally occurring isotopes Stable isotope? • Inside gray area? Stable isotope • On edge? Radioactive • Outside? Isotope not stable Not an element • What is an element? – Isotope detected? – Element 118 discovery last fall – Lasted 0.05 milliseconds > 10+15 s 10+10 s 10+07 s 10+05 s 10+04 s 10+03 s 10+02 s 10+01 s 10+00 s 10-01 s 10-02 s 10-03 s 10-04 s 10-05 s 10-06 s 10-07 s 10-15 s < 10-15 s 24 12 Mg Nd p = 92 n = 60 Ir p = 118 n = 77 Br p = 35 n = 55 152 60 195 77 90 35 p = 12 n = 12 238 92 p = 92 n = 146 191 77 p = 77 n = 114 U Ir 24 12 Mg Nd p = 60 n = 92 Ir p = 77 n =118 Br p = 35 n = 55 152 60 195 77 90 35 p = 12 n = 12 238 92 p = 92 n = 146 191 77 p = 77 n = 114 U Ir Worksheet 6. Imagine a chemist was trying to create an atom with 60 protons and a mass number of 155. Would this be possible? Why or why not? 7. Where on the graph would you expect the other isotopes of magnesium to be located (magnesium-25 and magnesium-26)? Explain. Worksheet 8. If an element had 90 protons, how many neutrons would be a good number for it to have in order to be considered a stable element? What element would this be? 9. What do you suppose that little island of gray on the graph represents? Check-In • Use your graph to determine how many neutrons you would need to make a stable element with 75 protons. • How many neutrons would make a radioactive element with 75 protons? Answer to #10 162 63 Eu No 75 33 As Yes 112 56 Ba Yes 260 88 Ra No 300 115 ???? Yes Notes • Radioactive elements lose pieces of the nucleus over time. • Atoms that exist for a long time are called stable. • Radioactive atoms disappear over time and are called unstable. • Any isotope that lasts long enough to be detected and measured qualifies as an element –Element 118 discovery last fall –0.05 milliseconds