17.2 Masses of Atoms
advertisement

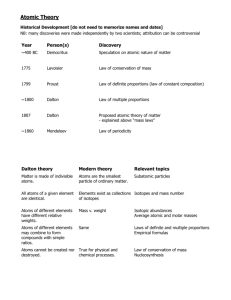
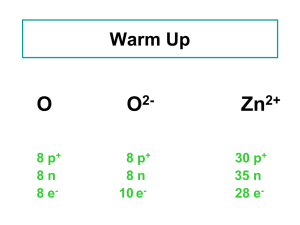
17.2 Masses of Atoms Atomic Mass The nucleus contains most of the mass of the atom bc P and N are far more massive than E. P & N are about the same size. Atomic mass Every P & N is about 1,836 times greater than the mass of one E. That means that the E’s mass is so small that it is considered negligible when finding the mass of an atom. Atomic Mass The unit of measurement used for atomic particles is the atomic mass unit (amu). The mass of a proton or a neutron is almost equal to 1 amu. The atomic mass unit is defined as onetwelfth the mass of a carbon atom containing six protons and six neutrons. Protons Identify the Element The # of P’s tell you what type of atom you have. Ex: every Carbon atom has 6 protons Ex: every atom with 6 protons is a Carbon atom The # of P in an atom is equal to a number called the atomic number. Atomic Number This means that if you are given… The name of the element, The number of P in the element The atomic number of the element …then, you can determine the other two. Mass Number The mass number of an atom is the sum of the number of P and the number of N in the nucleus of an atom. If you know the mass number and the atomic number of an atom, you can calculate the number of neutrons. Number of neutrons = mass # - atomic # Isotopes Not all the atoms of an element have the same number of Neutrons. Atoms of the same element that have different numbers of neutrons are called isotopes. Identifying Isotopes Boron isotopes shown BC the # of N is different in the isotopes, the mass numbers are different too. You use the name of the Element followed by the mass number of the isotope to identify each isotope Boron-10 Boron-11 Identifying Isotopes The average atomic mass of an element is the weighted-average mass of the mixture of its isotopes. EX: 4 out of 5 atoms of boron are boron-11 and one out of 5 is boron-10. 4/5 (11 amu) + 1/5 (10 amu) = 10.8 amu If the average atomic mass is not a whole number, that’s an indication that it has isotopes in nature. Isotope Notation- Add to Notes Isotopes can be notated in two different ways