Isotope PPT - MrsPage.com
advertisement
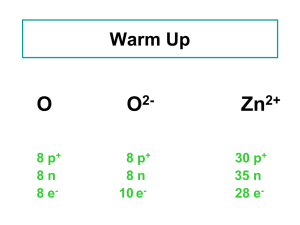
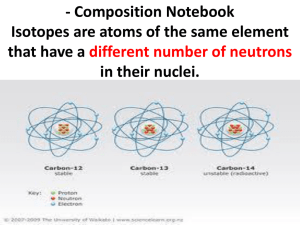
ATOMS & ISOTOPES Mrs. Page Chemistry10 Parts of presentation modified from a PowerPoint presentation prepared by J. Crelling, Southern Illinois University LEARNING OBJECTIVES • You will be able to define what an isotope is. • You will be able to determine the number of protons, neutrons, and electrons in different isotopes of the same element. • You will understand that atomic mass • You will understand what radioactivity is the average of the naturally occurring isotopes of an element. • You will be able to give examples of how radioisotopes are used in industry and medicine. ATOMS Contain protons, neutrons, and electrons Protons & neutrons are found in the nucleus The nucleus contains most of the mass of an atom Electrons are distributed around the nucleus in energy levels/shells/orbitals (which make up the electron cloud) The outermost electrons in the shell farthest from the nucleus are called valence electrons http://www.universetoday.com/ ELECTRONS First subatomic particle discovered 1897 J.J. Thomson used the cathode ray tube to discover the electron Has a negative charge (-1) Mass = 9.110 x 10-28 g (0.0005 amu) Electrons are located in energy levels which make up the electron cloud Electrons in the outermost energy level are called valence electrons Valence electrons are responsible the for the reactivity of an atom. PROTON Observed by E. Goldstein in 1896 Rutherford is given credit for showing that atoms contain both negatively and positively charged particles (gold foil experiment) Has a charge of +1 Relative Mass of 1 AMU (1.673 x 10-24 g) NEUTRON Third major subatomic particle discovered (1932 James Chadwick) No charge (neutral) Relative Mass of 1 AMU (1.675 x 10-24 g) ISOTOPES Atoms of the same element have the same atomic number (# of protons) and the same chemical properties. However, atoms of the same element may have different numbers of neutrons (and therefore different atomic mass) Isotopes are atoms of the same element having different number of neutrons NATURALLY OCCURRING ISOTOPES Every element has naturally occurring isotopes Hydrogen Protium has 3 naturally occurring isotopes is the most abundant isotope of hydrogen (99.985%) has 1 proton, 0 neutrons, and 1 electron Deuterium (0.015%) has 1 proton, 1 neutron, and 1 electron Tritium (0.0001% ?) has 1 proton, 2 neutrons, and 1 electron ISOTOPE EXAMPLE 35Cl 37Cl 17 17 chlorine - 35 chlorine - 37 ISOTOPES OF CARBON Naturally occurring carbon consists of three isotopes, 12C, 13C, and 14C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12C 6 13C 6 14C 6 #P _______ _______ _______ #N _______ _______ _______ #E _______ _______ _______ SOLUTION 12C 6 13C 6 14C 6 #P __6___ _ 6___ ___6___ #N __6___ _ _7___ ___8___ #E __6___ _ 6___ ___6___ ZINC PROBLEM An atom of zinc has a mass number of 65. A. Number of protons in the zinc atom B. Number of neutrons in the zinc atom C. What is the mass number of a zinc isotope with 37 neutrons? ATOMIC MASS Na 22.99 Listed on the periodic table Gives the mass of “average” atom of each element compared to 12C Average atom based on all the isotopes and their abundance % Atomic mass is not a whole number … mass number is a whole number CALCULATING ATOMIC MASS Percent(%) abundance of isotopes Mass of each isotope of that element Weighted average = mass isotope1(%) + mass isotope2(%) + … 100 100 ATOMIC MASS OF MAGNESIUM Isotopes Mass of Isotope 24Mg = 24.0 amu 25Mg = 25.0 amu 26Mg = 26.0 amu Abundance 78.70% 10.13% 11.17% (24)(.787) + (25)(.1013) + 26(.1117) = 18.888 + 2.5325 + 2.9042 = 24.3 amu ISOTOPES Two Categories Unstable – isotopes that continuously and spontaneously break down/decay in other lower atomic weight isotopes Stable – isotopes that do not naturally decay but can exist in natural materials in differing proportions USES OF RADIOISOTOPES Carbon 14 Dating (Geologic Time) When the organism dies it stops taking in 14C which disappears as it decays to 14N Americum-241 Used in smoke detectors Cesuim-137 Used to treat cancerous tumors Californium-252 Used to inspect luggage for explosives Cobalt-60 Used to sterilize surgical equipment Iodine-123 Used to treat thyroid disorders Plutonium-238 Used to power NASA spaceships