Chemistry lecture notes
advertisement

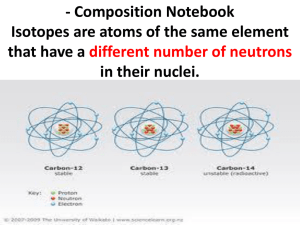
Lecture 1 Dr: Sahar Mousa Electrons and nuclei The Familiar Planetary Model of the atom was proposed by Rutherford in 1912 All the mass of an atom was concentrated in the positively charged (+) Nucleus. Electrons have Negatively charged (-). electrostatic force: it’s a force required to attracted the electrons to the nucleus. Nuclear structure Atomic number (Z) ,is the no. of protons and also the no. of electrons in a neutral atom. Mass number,is the no.of protons + the no. of neutrons The lightest atomic nucleus (hydrogen) is 1830 times more massive than an electron. The size of a nucleus is around 10−15 m (1 fm) Nuclear structure Nuclei contain positively charged Protons and uncharged Neutrons Protons and neutrons are held together by an attraction force (strong interaction). An electrostatic ruplsion between protons occurs inside the nucleus The balance of the two forces controls some important features of nuclear stability Nuclear structure The lighter nuclei are generally stable with approximately equal no. of protons and neutrons as O16 andC12. The heavier ones have higher proportion of neutrons as pb 208. As Z increases the electro static repulsion comes to be dominate. There is a limit to the number of stable nuclei. All elements beyond Bi (z=83)being radioactive. • Magic number The nuclei with even no. of either protons or neutrons ( or both) are generally more stable than ones with odd no. O16 and Pb208 are example of nuclei with magic no. -certain magic numbers of protons or neutrons, which give extra stability Isotopes are atoms of the same element that have a different number of neutrons. Therefore, isotopes have the following characteristics: Isotopes have the same atomic number (same number of protons), but a different atomic mass number (a different number of neutrons). Isotopes behave the same chemically, because they are the same element. The only difference is that one is heavier than the other, because of the additional neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon. Carbon-12 has 6 neutrons; carbon14 has 8 neutrons. Isotopes Some elements have: only one stable isotope (e.g. 19F, 27Al, 31P). others may have several (e.g. 1H and 2H, the latter also being called deuterium, 12C and 13C). Molar mass is also known as Relative atomic mass (RAM) is determine by the Proportions (Mixture of the isotopes of an element). Isotopes NMR (nuclear magnetic resonance ) technique depend on NMR of H1 and C13. All elements have unstable radioactive isotopes, some of these occur Naturally and the other can be mad e artificially. Importance and uses of isotopes (students) Radioactivity Radioactive decay: It is a process whereby unstable nuclei change into more stable ones by emitting particles of different kinds as Alpha, beta and gamma (α, β and γ) radiation Radioactivity Gamma radiation, is high energy electro magnetic radiation accompanies alpha and beta decays. Half-life is the time taken for half of a sample to decay. It can vary from a fraction of a second to billions of years. All elements beyond Bi (z=83) are radioactive and non beyond U(z=92) occur naturally on Earth. Radioactivity Spontaneous fission It occurs for heavy elements ,where the nucleus splits into two fragments of similar mass.