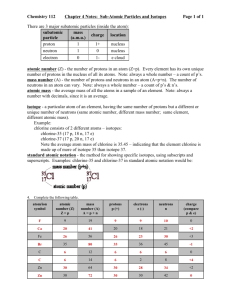
Particles of Atoms
advertisement
HOW DO YOU DETERMINE THE AMOUNT OF SUBATOMIC PARTICLES IN AN ATOM? IT BEGINS WITH THE PERIODIC TABLE An arrangement of elements in which the elements are separated based on properties DISTINGUISHING ELEMENTS The atomic number of every element represents the number of protons in the nucleus of an atom of that element. MASS NUMBER The total number of protons and neutrons in an atom. Number of neutrons = mass number – atomic number Chemical Symbol Shorthand Notation http://www.google.com/imgres?imgurl=http://www.hibbing.edu/chem/V.08/isotope_symbols_1.jpg&imgrefurl=http://www.rapidgraphs.com/qwr.php%3Fq%3Disotopenotation&usg=__12aXBa5PuDqU9mdbBqV92mGRrt0=&h=344&w=514&sz=40&hl=en&start=18&zoom=1&tbnid=fj4qDT6dfXQW2M:&tbnh=105&tbnw=157&ei=9lZcTYD5O5GSgQfB44SmDQ&prev=/images%3Fq%3 Dshorthand%2Bnotation%2Bfor%2Bchemical%2Bsymbols%26um%3D1%26hl%3Den%26rlz%3D1T4ADRA_enUS418US418%26biw%3D1259%26bih%3D560%26tbs%3Disch:1&um=1&itbs=1&iact=rc&dur=445&oei= 71ZcTZmxJoHegQenwcD-DQ&page=2&ndsp=21&ved=1t:429,r:12,s:18&tx=99&ty=18 WHAT ABOUT ELECTRONS? Electrons in an atom are equal to the number of protons. FORCE OF THE NUCLEUS The nucleus is made of p + n 2 protons are close together there is a strong attraction Nuclear Force ISOTOPES Atoms (of the same element) that have the same number of protons but a different number of neutrons. Do Isotopes have different mass numbers? COMMON ISOTOPES Hydrogen Hydrogen-1 (Hydrogen) Hydrogen-2 (Deuterium) Used as a moderator in nuclear reactors Hydrogen-3 Used (Tritium) in the production of Hydrogen bombs PRACTICE! There are 3 isotopes of Oxygen. Oxygen-16, Oxygen-17 and Oxygen 18. Write each in shorthand notation. There are 3 isotopes of Chromium. Chromium50, Chromium-52 and Chromium-53. How many neutrons are in each isotope? ATOMIC MASS UNITS One twelfth of the mass of a carbon-12 atom IF THERE ARE ISOTOPES HOW DO YOU CALCULATE 1 ATOMIC MASS? Calculate a weighted average Not You each isotope is as common as the next need to know the mass of each isotope and its natural abundance.