Hybrid Orbitals
advertisement

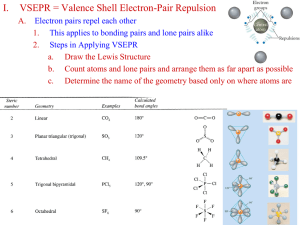
Word Splash – Write a few sentences about how the following terms are related Hybridization Additional Notes Structure and Bonding – sp3 orbitals • Look at methane, CH4 • We must take a look at the 2s and 2p orbitals of carbon • Carbon has two core electrons, and 4 valence • Look at the ground state electron configuration Structure and Bonding - sp3 orbitals Structure and Bonding - sp3 orbitals • Using the ground state configuration, carbon should only form two bonds • Bc there are only two unpaired electrons: • This is NOT STABLE! NO OCTET! • Does not work! Structure and Bonding - sp3 orbitals • Second possibility: with energy, promote an electron in 2s to the empty 2p orbital • Still not good description! This means carbon would form two types of bonds: 3 bonds with the 2p orbitals and 1 bond with the 2s orbital Structure and Bonding - sp3 orbitals • Evidence shows that the four bonds in methane are identical • We do not use pure s and pure p orbitals, instead we use hybrid of the two types of orbitals Structure and Bonding – sp3 orbitals • Hybridization of the 2s and three 2p orbitals produced four hybrid orbitals: Structure and Bonding – sp3 orbitals • Each bond in CH4 is formed by an overlap of one sp3 orbital of carbon with a 1s orbital from hydrogen Structure and Bonding - sp2 orbitals • One 2s orbital and two 2p orbitals form three sp2 hybrid orbitals, leaving one of the 2p orbitals unhybridized Structure and Bonding - sp2 orbitals • Let’s look at ethene, C2H4 Structure and Bonding – sp orbitals • Let’s look at ethyne, C2H2 Summary Summary