Zumdahl`s Chapter 9
advertisement

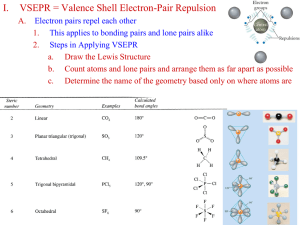
Zumdahl’s Chapter 9 Covalent Bonding Orbitals Chapter Contents Hybridization and LE dnspm Molecular Orbitals Bond Order Bonding in Homonuclear Diatomics Paramagnetism Heteronuclear Bonding LE + MO Atomic Orbital Hybridization VSEPR postulates repulsion geometries, but are atomic wavefunctions flexible enough to supply? A key to wave mechanics is superposition, creating new waves from interference of old ones. Degenerate (same energy) wavefunctions can mix arbitrarily to give new degenerate sets. # # If energy lowering is possible, even near-degenerate (similar energy) sets of can mix to new sets. The Joys of Promotion Minimization of electron repulsion is the motive for mixing a set of AOs (Atomic Orbitals) that produces new AOs in the VSEPR directions. In addition, paired electrons in AOs are already satisfied and need no stinking bonds, but if these partners are split up, more bonding is possible, at the bargain expense of promotion to higher energy orbitals. Extra bond energies pay it back. Carbon Wins Big The electronic configuration of carbon is [He] 2s2 2p2 or [He] [] [ ] [ ] [ ] As such, only CH2 or two valence bonds are possible for carbon. Not for long. Using the energy accessible from bond formation, carbon promotes an s e– to a p orbital [He] 2s1 2p3 or [He] [ ] [ ] [ ] [ ] Making CH4 and other four valence molecules. Blending the l out of it. Promotion is necessary but not sufficient; we must still mix s+px+py+pz in ways like VSEPR. The electron density follows the new orbital directions and the nuclei obey the bonding geometry. We needn’t blend all of the available orbitals! We can mix spx and leave py and pz for lone pair. That would give us linear XAX bonding. And that results from constructive interference in . Wave Mixture Geometries 2s + Bonding Direction 2px = Node shifted off x=0 plane. sp Hybridization Starting with two AOs, mixing must generate no more than nor no fewer than two hybrids. The s + px combination points up the x-axis while the s – px combination points down the x-axis. Together they give linear XAX molecules (BeF2). Also, the sp hybrid is elongated in its bonding direction for better penetration and lower energy. Trigonal Planar Hybridization, sp2 and mixed with and 2 other combinations gives 2 sp Hybridization As with sp, the leftover p orbital(s) are available for electrons as either lone pairs or bonds. In contrast, the single bonds created with the hybrids are called bonds, where is the Greek s and is the Greek p. All 3 sp2 together look like: Truly 120° apart If your browser supports VRML, try http://www.chm.davidson.edu/vrml/ao/ for details of hybrid orbitals. sp3 Tetrahedral Hybridization The sp2 are even more penetrating than the sp but less so than sp3. The more the p character, the more directional the hybrid. Duhh. Carbon can hybridize sp3, but so can N and O; the difference is in how many hybrids have only one electron. Those bond; the others lone pair. The four sp3 together look like: And the angle is now cos–1(–1/3) = 109.43° Hybridization Beyond n sp The sp3 hybrid orbitals permit a valence of up to 4 and the expected octet. Violating the octet demands incorporation of d orbitals as in dsp3, d2sp3, etc. and dx²–y² They use dz² Trigonal Bipyramidal 3 dsp Mixing dz² with sp3 gives five orbitals, two of which are axial and three equatorial. Only one each of the two kinds are shown here, but the other axial is just down the Z while the other two equatorials are around Z at 120° to the one shown. Octahedral d²sp³ Mixing dx²–y² with the five dsp³ gives the six identical d²sp³ orbitals in Cartesian directions. This picture is merely diagrammatic; it is not an accurate representation of the d²sp³ wavefunctions. (It is a group of spheres and cones.) Still, if your browser is equipped with VRML, you can play with my first 3d world construction at http://www.utdallas.edu/~parr/chm1315/d2sp3.wrl How Chemists Use Hybrids Hybrid orbitals are great shorthand notations for building up a molecule’s geometry center by center … part of every molecular model kit. The skeletal structure so developed is called the skeleton because in-line overlap of adjacent bonding hybrids are cylindrically symmetrical about the internuclear axis and thus have no (axial) angular momentum (like s for an atom). and the remainders The skeleton sets hybridization for each of the molecule’s central atoms. Bonded to 3 things? No lone pair? You are sp2! I not only expect 120° bonds from you but also an unhybridized p orbital to that plane. Since the rest of your octet isn’t a lone pair, you must be bonding to one or more of your partners. Bonded to 3 with a lone pair? You’re sp3! I expect 109.43° bond angles or there abouts. bonds? Piece of cake. Unhybridized p orbitals on adjacent bonding centers can overlap (inefficiently) sideways. Density not cylindrically symmetric (like ) but does allow for buildup between nuclei (off line-of-centers). bonds weaker than but add 1 to the bond order. Off axis, they’re vulnerable to chemical attack; bonds are reactive while are relatively inert. “Unsaturated” fats have ’s to permit metabolic degrade. Vitamin B12 sp CN ligand sp2 C=O d2sp3 Co sp3 sp3 SO4 Vitamin B12 with its multiple bonds Molecular Orbital (MO) Theory Electrons don’t ignore all other nuclei beyond the adjacent bonding pair. They’re really global. Instead of building molecules atom by atom, we’ll pour electrons onto a nuclear skeleton. Hess assures us that when all the electrons are finally present, the (binding) energies will be the same either way. So how do electrons respond differently this way? Add electrons to proton framework They see a wavefunction that spans molecule! First approximation model to that is LCAO, Linear Combination of Atomic Orbitals. Study the diatomics for simplicity. The advantages to MO will become apparent even there. Thus, H2’s MOs are LC of 1sA and 1sB where A and B are the labels for the two hydrogen atoms. 1s 1sA + 1sB while 1s* 1sA – 1sB (2 in; 2 out) 1s H2 A MO also builds density between bonded nuclei. B Fortunately, this MO holds both electrons. See http://www.chm.davidson.edu/vrml/mo/h2/h2.html 1s* H2 MO A Electron density vacates region between nuclei! B Any electron in this antibonding MO reduces BO by ½. Bond Order (BO) in MO MO’s come in constructive (e.g., 1s) and destructive (e.g., 1s*) combinations as regards the internuclear region. Since they must mimic lone pairs as well, there are nonbonding MO’s, but they do not influence BO. BO = ½ ( electrons MO – electrons MO* ) First surprising consequence: H2+ has BO = ½ A stable one-electron bond is possible. Correlates with Diss. E. and negatively with Bond R. Using p AO’s If internuclear axis is Z, then 2pZA – 2pZB binds and is called 2pz. The “+” combo anti*binds. More interesting are PX and PY which combine off the internuclear axis as MO’s. 2pX* 2pXA – 2pXB for example. Note that some combinations are meaningless because they do not overlap in bonding regions: e.g., 2pXA + 2pYB produces no MO. Degenerate MO’s The energies of 2pX and 2pY are identical. Hund’s Rule applies to MO’s just as it did AO’s. 1s2 1s*2 2s2 2s*2 2pZ2 2pX1 (C2+) is followed by 1s2 1s*2 2s2 2s*2 2pZ2 2pX1 2pY1 (C2) Implying not only C=C but also or a paramagnetic C2 diatom. Decline of Bond Order The pinnacle of A2 (2nd row generic diatom) comes at N2 with electronic configuration: [Be2] 2pZ2 2pX2 2pY2 and bond order 3, NN. We’ve run out of bonding MO’s of the 2 shell. Starting with O2, antibonding highest occupied MO’s (HOMO) diminish BO. [Be2] 2pZ2 2pX2 2pY2 2pX*1 2pY*1 Only bond order 2 but paramagnetic. See http://www.chem.technion.ac.il/ElBookOrb/molecule.htm Formic Acid, HCO2H Lewis Structure would have resonance in the conjugate base with the C-O bonds at 1.5 order. MO generates this naturally by mixing 2pX from both oxygens and the carbon to create: An example of the delocalized nature of bonding. bonding is better described as local. Their mixing generates these double bonds. Benzene, a textbook delocalization After hybridizing sp2 for the skeleton, Yes, you can build MO’s from hybridized AO’s. The 6 leftover pX orbitals mix to give global MO’s the plane of the nuclei. Before mixing they are: Evidence of delocalized electrons comes from benzene’s magnetic “ring current.” C6H6 Bonding After mixing, six new MO’s arise, 3 bonding and 3 antibonding. Best case at top, and worst case at bottom. The 6 electrons from each carbon’s p pair up in the 3 bonding ’s. Knowing Where the Electrons are is POWER PC Spartan