OrbitalOverlapWorksheet
advertisement

Created by Vanessa P. McCaffrey (vmccaffrey@albion.edu) and posted on VIPEr in May 2014. Copyright Vanessa P. McCaffrey, 2014. This work is licensed under the
Creative Commons Attribution Non-commercial Share Alike License. To view a copy of this license visit {http://creativecommons.org/licenses/by-nc-sa/3.0/}.
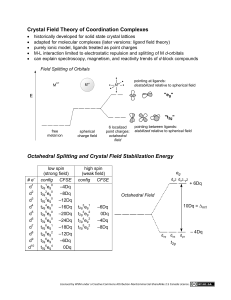
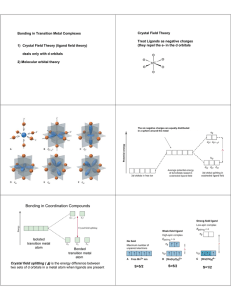
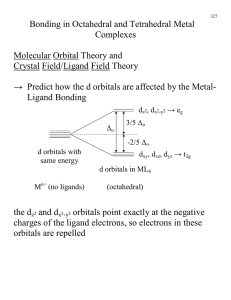
This exercise is designed to familiarize you with the possible bonding interactions of a variety of different orbitals, and in particular dorbitals. You will need to draw the indicated orbitals for a hypothetical A-B compound. For each of the combinations below, assume
that the orbital given in the column is on the left and the row is on the right.
a. sketch the orbitals with the appropriate relative orientation (assume that the z-axis is the bonding axis and horizontal within
the plane of the paper). Generally, the x-axis is vertical within the paper, but you may reorient if necessary. Label your axes!!
b. If there is a net bonding interaction for the interaction of the two orbitals, indicate the overlap and classify the type of
interaction (sigma, pi, delta).
c. If no net bonding interaction takes place, write “NO INTERACTION” under the drawing of the orbitals.
A/B
s
px
py
pz
s
px
py
pz
dxy
dyz
dxz
dx2-y2
dz2
Created by Vanessa P. McCaffrey (vmccaffrey@albion.edu) and posted on VIPEr in May 2014. Copyright Vanessa P. McCaffrey, 2014. This work is licensed under the
Creative Commons Attribution Non-commercial Share Alike License. To view a copy of this license visit {http://creativecommons.org/licenses/by-nc-sa/3.0/}.
s
dxy
dyz
dxz
dx2-y2
dz2
px
py
pz
dxy
dyz
dxz
dx2-y2
dz2