Supplementary Material to "Experimental
advertisement
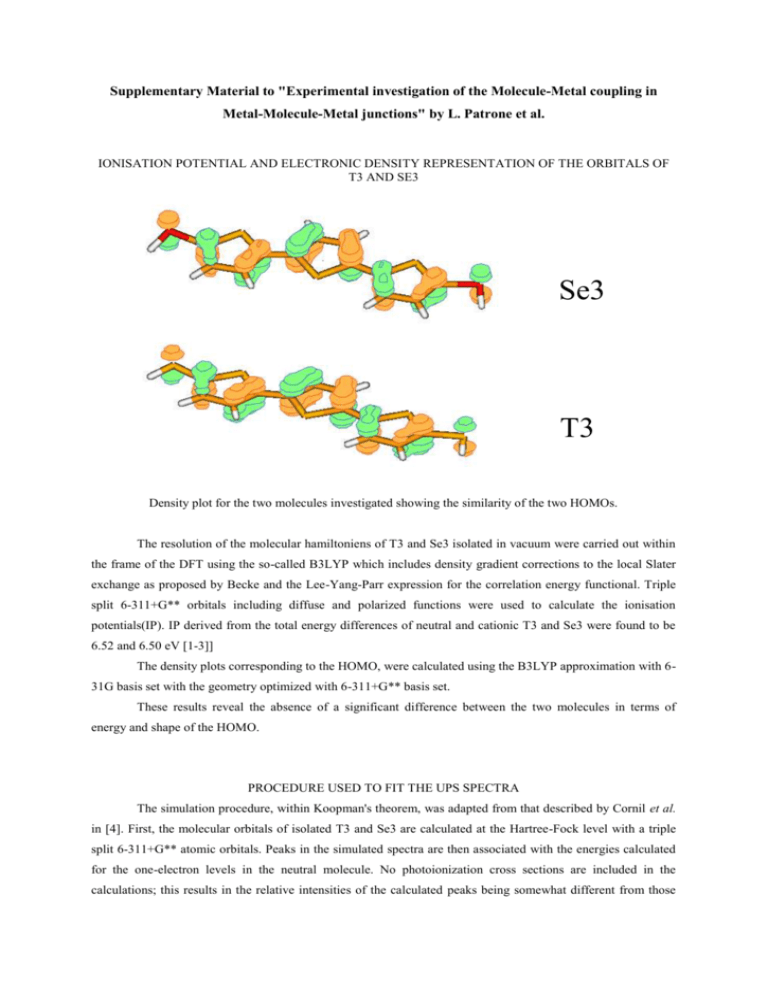
Supplementary Material to "Experimental investigation of the Molecule-Metal coupling in Metal-Molecule-Metal junctions" by L. Patrone et al. IONISATION POTENTIAL AND ELECTRONIC DENSITY REPRESENTATION OF THE ORBITALS OF T3 AND SE3 Se3 T3 Density plot for the two molecules investigated showing the similarity of the two HOMOs. The resolution of the molecular hamiltoniens of T3 and Se3 isolated in vacuum were carried out within the frame of the DFT using the so-called B3LYP which includes density gradient corrections to the local Slater exchange as proposed by Becke and the Lee-Yang-Parr expression for the correlation energy functional. Triple split 6-311+G** orbitals including diffuse and polarized functions were used to calculate the ionisation potentials(IP). IP derived from the total energy differences of neutral and cationic T3 and Se3 were found to be 6.52 and 6.50 eV [1-3]] The density plots corresponding to the HOMO, were calculated using the B3LYP approximation with 631G basis set with the geometry optimized with 6-311+G** basis set. These results reveal the absence of a significant difference between the two molecules in terms of energy and shape of the HOMO. PROCEDURE USED TO FIT THE UPS SPECTRA The simulation procedure, within Koopman's theorem, was adapted from that described by Cornil et al. in [4]. First, the molecular orbitals of isolated T3 and Se3 are calculated at the Hartree-Fock level with a triple split 6-311+G** atomic orbitals. Peaks in the simulated spectra are then associated with the energies calculated for the one-electron levels in the neutral molecule. No photoionization cross sections are included in the calculations; this results in the relative intensities of the calculated peaks being somewhat different from those observed in the experimental spectra. Finally, the spectra are convoluted with Gaussian functions with a full width at half-maximum of 0.8 eV. Two systematic corrections are applied as discussed in [4]. First, the energy scale is corrected by a factor 1/1.375 to account for the absence of correlation effects and second, the peaks are shifted rigidly so that the main peaks fit: as a result, the lower energy peak maximum is located at respectively 1.3 and 1.6 eV from the Fermi level of gold for Se3 and T3. DETAILS OF THE ESQC CALCULATION The Sulfur(Selenium) atom sits on a 3 fold symmetry, fcc or hcp, site on a Au(111) slab on top of which the molecule was positioned with a tilted angle of 30°. The tip apex was positioned above the last atom on the top of the molecule. The total number of atoms in the model is 280 The Au(111) surface is described by a two layers slab of 144 atoms and the tip by a <111> oriented Au cluster of 35 atoms supported by a layer of 72 Au atoms. All Au atoms were described by only the 6s orbitals with standard STO (Slater type orbitals) parameters. However for the first Au layer at the surface and the atom at the tip apex end, parameters were optimized by using a double zeta basis to satisfy the classical tunnel current decrease slope in the vacuum (one order of magnitude for one angstrom at large tip-sample separation). For molecules, only valence orbitals with classical parameters were used for H, C, O, S and Se atoms (Au(bulk) 6s -10.92 2.20 1.0; Au(surf) 6s -10.92 2.00 0.60 1.05 0.40; Au(apex) 6s -10.92 2.00 0.9900 0.835 0.010; S 3s -20.0 1.817 1.0; 3p -13.3 1.817 1.0; Se 4s -20.5 2.070 1.0; 4p -14.4 2.070 1.0). The total number of atomic orbitals is 340 with 196 orbitals in the defect. [1] [2] [3] [4] A.D. Becke, Phys. Rev. A 38, 3098 (1988). W. Y. C.L. Lee, R.G. Parr, Phys. Rev. B 37 (1988) 785. M. J. Frisch, et al.,, (Gaussian Inc, Pittsburgh PA, 2001). J. Cornil, et al., Chem. Mater. 11, 2436 (1999).