Bonding orbitals
advertisement

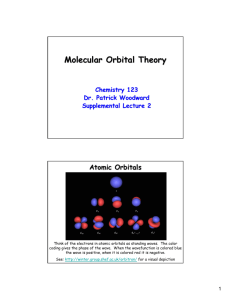
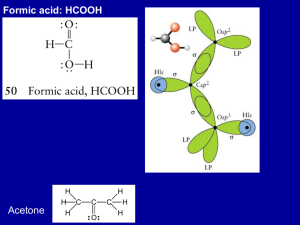
Molecular Orbital Theory LCAO-MO = linear combination of atomic orbitals ψ 1 = c 1 φ1 + c 2 φ2 ψ 2 = c 1 φ1 - c 2 φ2 Add and subtract amplitudes of atomic orbitals to make molecular orbitals Just like making hybrid orbitals, but AO’s come from different atoms Bonding orbitals: Electrons have high probability of being between nuclei (lower energy) Orbital overlap determines bonding energy Weak overlap => weak interaction (bonding & antibonding MO energy same as AO’s Strong overlap => lowers energy of bonding MO, raises energy of antibonding MO Bonding and antibonding orbital energies in H2 We typically draw MO energy level diagrams at the equilibrium bond distance MO energy level diagram for H2 (H2+, HHe, He2, …) α = Coulomb integral => ionization energy of electron in atomic orbital, e.g., H1s β = Exchange integral => energy difference between AO and bonding orbital S = Overlap integral, S12 = ∫φ1*φ2dτ MO diagram for a polar bond (e.g., in HCl) α values are different because of electronegativity difference between H and Cl Larger difference between bonding and antibonding orbital energies Bonding orbital closer in energy to Cl 3pz AO = > bond has more “Cl character” MO diagram for an ionic bond (e.g., in Na+F-) Larger energy difference Bonding electron pair is localized on the F atom Excited state is Na0F0 Summary of MO theory so far: • Add and subtract AO wavefunctions to make MOs. # of AOs = # of MOs. • More nodes → higher energy MO • Bond order = ½ ( # of bonding electrons - # of antibonding electrons) • Bond polarity emerges in the MO picture as orbital “character.” • AOs that are far apart in energy do not interact much when they combine to make MOs. Orbital Symmetry AO’s of different symmetries (in the point group of the molecule) do not interact Greatly simplifies the problem of constructing MO’s for complex molecules MO diagram for HCl molecule Cl 2px and 2py orbitals have π symmetry – no interaction with σ symmetry orbitals Cl 3s is too low in energy to interact => nonbonding electron pair 8 electrons => 1 bond + 3 lone pairs (same result as valence bond picture) σ, π, and δ orbitals in inorganic compounds Face-to-face overlap of d-orbitals => δ bond e.g., in [Re2Cl8]2− σ and π bonding in metal d-orbital complexes Early transition metal Empty d-orbital Late transition metal Filled d-orbital Ligand acts as a σ donor (= Lewis base), empty d-orbital is σ acceptor (Lewis acid) Ligands can also act as π donors or π acceptors MO diagram for 2nd row diatomic molecules Li2, Be2, B2, C2, N2 O2, F2 Fill up MOs in Aufbau order O2 = 12 e = double bond, 2 unpaired electrons (paramagnetic) B2 , C2 ? O2 MO diagram & orbitals π-bonding: 2nd row vs. 3rd (4th, 5th, 6th) rows Ethylene: Stable molecule, doesn't polymerize without a catalyst. Silylene: Never isolated, spontaneously polymerizes. The large Ne core of Si atoms inhibits sideways overlap of 3p orbitals → weak π-bond N can make π-bonds, so N2 has a very strong triple bond and is a relatively inert diatomic gas “RTV” silicone polymer (4 single bonds to Si) vs. acetone (C=O double bond)