Chapter 6 - Evangel University
advertisement

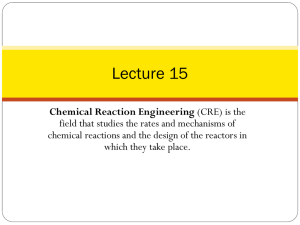
Mary K. Campbell Shawn O. Farrell http://academic.cengage.com/chemistry/campbell Chapter Six The Behavior of Proteins: Enzymes Paul D. Adams • University of Arkansas Enzyme Catalysis • Enzyme: a _____________________ • with the exception of some __________ that catalyze their own splicing (Section 10.4), all enzymes are proteins (???) • enzymes can increase the rate of a rxn by a factor of up to 1020 over an uncatalyzed rxn • some enzymes are so specific that they catalyze the rsn of only one stereoisomer; others catalyze a family of similar rxns • The rate of a reaction depends on its activation energy, DG°‡ • an enzyme provides an alternative pathway with a ______________________________ Enzyme Catalysis (Cont’d) • For a reaction taking place at constant temperature and pressure, e.g., in the body A B • the change in __________________________ is DG° = DH° - TDS° • Difference in energies between initial state and final state DG° = RT l n Keq • The change in free energy is related to the equilibrium constant, Keq, for the reaction by Enzyme Catalysis (Cont’d) • Consider the reaction H2O2 → H2O + O2 Temperature dependence of catalysis • Temperature can also “catalyze reaction” (increase rate) • This is dangerous, why? • Increasing temperature will lead to _______________ ______________________ Enzyme Kinetics • For the reaction A + B P • The rate of reaction is given by rate equation D[A] D[B] _ Rate = _ = Dt Dt = D[P] Dt Rate = k[A] f[B] g • Where k is a proportionality constant called the ___________________________________________ • ______________________________: the sum of the exponents in the rate equation: f+g Enzyme Kinetics (Cont’d) • Consider the reaction A + B C + D Whose rate equation is given by the expression Rate = k[A]1[B]1 • Determined experimentally, not from _______________ • The reaction is said to be first order in A, first order in B, and second order overall • Consider this reaction of glycogen with phosphate Gl ycogenn + HPO42- Gl ucose-1-phosphate + Glycogen n-1 1 Rate = k[Glycogen] [HPO42-]1 = k[Glycogen][HPO42-] How Enzymes bind to Substrate • In an enzyme-catalyzed reaction • ____________________________, S: a reactant • ______________________: the small portion of the enzyme surface where the substrate(s) becomes bound by noncovalent forces, e.g., hydrogen bonding, electrostatic attractions, van der Waals attractions E + S ES e nz ym e -substrate com ple x Binding Models • Two models have been developed to describe formation of the_____________________ complex • __________________ model: substrate binds to that portion of the enzyme with a complementary shape • _________________ model: binding of the substrate induces a change in the conformation of the enzyme that results in a complementary fit Two Modes of E-S Complex Formation Formation of Product An Example of Enzyme Catalysis ____________________ catalyzes • The selective hydrolysis of ___________________ where the ________ is contributed by _____ and ____ • It also catalyzes hydrolysis of the ____________ bonds An Example of Enzyme Catalysis (Cont’d) Non-Allosteric Enzyme Behavior Point at which the rate of reaction does not change, enzyme is __________________, maximum rate of reaction is reached ATCase: An Example of Allosteric Behavior • ____________ shape - characteristic of __________ • Again max velocity reached, but different mechanism Michaelis-Menten Kinetics Initial rate of an enzyme-catalyzed rxn vs [S] Michaelis-Menten Model • For an enzyme-catalyzed reaction E + S k1 k-1 ES k2 P • The rates of formation and breakdown of ES are given by these equations rate of formation of ES = 1k[E][S] rate of breakdown of ES = k -1 [ES] + k2[ES] • At steady state k1[E][S] = k-1[ES] + k2[ES] Michaelis-Menten Model (Cont’d) • When ______________is reached, the concentration of free enzyme is the total minus that bound in ES [E] = [E]T - [ES] • Substituting for the concentration of free enzyme and collecting all rate constants in one term gives ([E]T - [ES]) [S] = [ES] k-1 + k2 = KM k1 • KM is called the ____________________________ Michaelis-Menten Model (Cont’d) • It is now possible to solve for the concentration of the enzyme-substrate complex, [ES] [E]T [S ] - [ES ][S ] = KM [ES ] [E]T [S ] - [ES ][S ]= KM [ES ] [E]T [S ] = [ES ](KM + [S ]) • Or alternately [ES] [E] T [S] = KM + [S] Michaelis-Menten Model (Cont’d) • In the initial stages, formation of product depends only on the _______________________________________________ Vinit = k2 [ES] k 2[E]T [S] = KM + [S] • If substrate concentration is ________________________ is _______________________ [ES] = [E]T Vinit = Vmax = k2[E]T • Substituting k2[E]T = Vmax into the top equation gives Vinit = Vmax [S] KM + [S] Mi chae l is-Mente n e quation Michaelis-Menten Model (Cont’d) When _______________ the equation reduces to V= Vmax [S] KM + [S] = Vmax [S] [S] + [S] = Vmax 2 Linearizing The Michaelis-Menten Equation • Vmax is difficult to ___________________________________ • The equation for a hyperbola Vmax [S] V= KM + [S] (an equati on for a hype rbol a) • Can be transformed into the equation for a ________ by taking __________________________________ 1 V = 1 V = KM + [S ] Vmax [S ] = KM + Vmax [S ] KM 1 + Vmax [S ] Vmax [S] Vmax [S ] Lineweaver-Burk Plot • The _______________________ plot has the form y = mx + b, and is the formula for a straight line 1 V KM = Vmax 1 • [S] + 1 Vmax y = • + b m x • a plot of 1/V versus 1/[S] will give a straight line with slope of _______________ and y intercept of _______________ • known as a __________________________________________ Lineweaver-Burk Plot (Cont’d) • KM is the ________________________________________ • the greater the value of KM, the ________ tightly S is bound to E • Vmax is the ___________________________________ Turnover Numbers • Vmax is related to the ___________________________ of enzyme: also called kcat V max turnover_ num ber kcat [ET ] Number of moles of substrate that react to form product _____________________________________________ Enzyme Inhibition • ____________ inhibitor: a substance that binds to an enzyme to inhibit it, but can be released • ____________________________ inhibitor: binds to the active (catalytic) site and blocks access to it by substrate • _____________________ inhibitor: binds to a site other than the active site; inhibits the enzyme by changing its conformation • ________________________inhibitor: a substance that causes inhibition that cannot be reversed • usually involves formation or breaking of covalent bonds to or on the enzyme Competitive Inhibition • Substrate competes with inhibitor for the active site; more substrate is required to reach a given reaction velocity EI I + E + S ES • We can write a dissociation constant, KI for EI EI I + E KI = [E][I] [EI] P Competitive Inhibition Competitive Inhibition y = • No i nhibi ti on 1 = KM V Vmax m • 1 S + x + 1 Vmax b In the pre sence of a compe ti ti ve inhi bitor 1 V y KM = Vmax = 1 + [I] KI 1 S + m • x + 1 Vmax b In a Lineweaver-Burk plot of 1/V vs 1/[S], the __________________ (and the x intercept) changes but the ______________________ does not change A Lineweaver-Burke Plot, Competitive Inhibition Noncompetitive Inhibition (Cont’d) • Several equilibria are involved +S E -I ES E + P -S +I -I +S EI +I ES I -S • The maximum velocity Vmax has the form V I max = V max 1 + [I]/K I Noncompetitive Inhibition (Cont’d) Lineweaver-Burke Plot, Noncompetitive Inhibition • Because the inhibitor does not interfere with ______________ to the active site, KM is ______________________ • Increasing substrate concentration ____________________ noncompetitive inhibition No i nhibi ti on 1 = KM • 1 V S Vmax y = m • x + 1 Vmax + b In the pre se nce of a noncompe titive i nhi bitor [I] 1 1 = KM 1 + [I] 1 + 1 + V S Vmax Vmax KI KI y = m • x+ b Lineweaver-Burke Plot, Noncompetitive Inhibition Other Types of Inhibition • _____________________ - inhibitor can bind to the ES complex but not to free enzyme; Vmax decreases and KM decreases. • __________________ - Similar to noncompetitive, but binding of I affects binding of S and vice versa.






![[S], K m](http://s2.studylib.net/store/data/005727372_1-436e65aa9ae986608afd0b1018baee47-300x300.png)