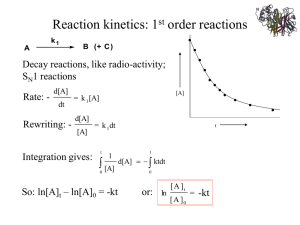
LAB 3 Enzyme Kinetics
advertisement

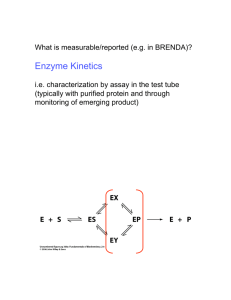
LAB 3 Enzyme Kinetics • Studying galactosidase activity at varying substrate concentrations in the presence and absence of an inhibitor • Michaelis-Menten enzyme kinetics • Lineweaver Burk plot Michaelis-Menten Enzyme Kinetics Steady-state Model: Formation of ES = Disappearance of ES k1[E][S] = k2[ES] + kcat[ES] Define a new constant, Km (Michaelis constant): Km = (k2 + kcat)/k1 Km reflects the affinity of the enzyme for the substrate: • low Km = high affinity • Km = [S] that produces V= 1/2Vmax The Michaelis-Menten Equation V= Vmax[S] Km + [S] 1/2Vmax Hyperbolic function Simplifying M & M analysis using a Lineweaver-Burk Plot • A double-reciprocal representation of V vs. [S] data: plot 1/V vs. 1/[S] • Take the inverse of both sides of the M-M equation to get the L-B equation, which specifies a line. M-M V= Inverse M-M Vmax[S] Km + [S] 1/V = (Km + [S]) / Vmax[S] Rearrange… L-B 1/V = 1/Vmax + Km/ Vmax (1/[S]) Determining Kinetic Parameters from an L-B Plot 1/V = 1/Vmax + Km/ Vmax (1/[S]) Last week’s experiments vs. today’s • Last week studied enzyme at saturating substrate concentrations, meaning there was so much substrate that the enzyme worked at Vmax all the time so we could determine specific activity. • Today, varying concentration of substrate to find out what happens when substrate is limiting. How strong of an affinity does the enzyme have for this substrate? Can determine the Michaelis constant, Km. TO DO TODAY • Vary substrate (ONPG) conc. over an 80-fold range to determine Km, the substrate conc. that gives V = 1/2 V max • Repeat above using a constant amount of an inhibitor (IPTG) to determine Km in the presence of the inhibitor. • Determine whether the inhibition is competitive or noncompetitive by comparing Km & Vmax -/+ IPTG on MichaelisMenten & Lineweaver-Burk plots. Competitive Inhibition No product formed • Inhibitor has a chemical structure similar to that of the substrate and competes for binding to the active site (can only bind free E). • Inhibition can be overcome by excess substrate. • Inhibitor increases Km but leaves Vmax unchanged. Noncompetitive Inhibition Diminished product formation • Inhibitor’s chemical structure may be totally different from that of the substrate. • Inhibitor binds a site on the enzyme that is distinct from the active site. • EI complex can bind substrate, but cannot transform it into product as efficiently as the uninhibited enzyme because of alterations in the shape/chemistry of the active site. • Inhibition cannot be overcome by excess substrate. • Inhibitor decreases Vmax but leaves Km unchanged. Michaelis-Menten Kinetics with Inhibition Lineweaver-Burk Plots with Inhibitors Ki • The dissociation constant for an inhibitor • Whereas Km reflects the affinity of an enzyme for its substrate, Ki reflects its affinity for binding to an inhibitor. • Method of calculating Ki depends on the type of inhibition. For competitive inhibition: Km +I = Km -I ( 1 + [I]/Ki) For noncompetitive inhibition: Vmax +I = Vmax -I / ( 1 + [I]/Ki) Two more types of inhibition 1. Mixed inhibition • Inhibitor can bind both E and ES, but with different affinities (different Kis). • Both Vmax and Km may change; if inhibitor binds E with higher affinity than ES, Vmax decreases and Km increases. 2. Uncompetitive inhibition (quite rare) • Inhibitor binds only ES (not free E). • Both Vmax and Km decrease by same factor. Substrates & an inhibitor of -galactosidase What kind of inhibitor is IPTG likely to be? TO DO TODAY • Assay the dilution of PF of beta-gal from last week that gave Abs of approx. 0.5 – (Remember it has been diluted 2/3 with glycerol) • DO NOT ADD ENZYME TO REAGENT BLANKS (TUBES #10 & 11) • DO NOT add the enzyme until timing starts • Time carefully, mix well • Do the assay twice, once without inhibitor and again with inhibitor (IPTG) • Make Michaelis-Menten and Lineweaver Burk plots using Excel following directions in appendix after Lab 3 in lab manual • Make sure you understand homework calculations—15 points Homework Calculations 15 points • Protein conc. (mg/ml) for PF using lab 2 result (adjusted for addition of glycerol) • ONPG & IPTG conc. in nmol/ml – Volume used = 2.4 ml – Stock ONPG (mol. wt 301.3) = 4 mg/ml – Stock IPTG (mol. wt 238.3) = 0.6 mg/ml • Velocity (Spec. Activity) – Units = nmol/min/mg protein – Volume used = 3.4 ml • Michaelis-Menten plot (ONPG is substrate) – without IPTG – with IPTG • Lineweaver-Burk plot – without IPTG – with IPTG • Ki, disassociation constant of Inhibitor




![[S], K m](http://s2.studylib.net/store/data/005727372_1-436e65aa9ae986608afd0b1018baee47-300x300.png)