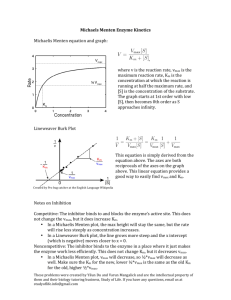
The V max and K m values of a certain enzyme can be measured by
advertisement

The Vmax and Km values of a certain enzyme can be measured by the double reciprocal plot. The plot provides a useful graphical method for analysis of the Michaelis–Menten equation. Taking the reciprocal gives The double reciprocal plot: 1/V0 vs 1/[S] Apply this to equation for a straight line When we plot versus Hanes–Woolf plot: A graphical representation of enzyme kinetics in which the ratio of the initial substrate concentration [S] to the reaction velocity v is plotted against [S]. Inversting and multiply by [S]: A perfect data will yield a straight line of slope 1/Vmax, a yintercept of Km/Vmax and an x-intercept of −Km. Hanes–Woolf plot Eadie–Hofstee Plot Model Is a graphical representation of enzyme kinetics in which reaction velocity is plotted as a function of the velocity vs. substrate concentration ratio: A plot of v vs v/[S] will yield Vmax as the yintercept, Vmax/Km as the x-intercept, and Km as the slope. Like other techniques that linearize the Michaelis–Menten equation, the Eadie-Hofstee plot was used historically for rapid identification of important kinetic terms like Km and Vmax, : invert and multiply with Vmax Eadie–Hofstee Plot Model The "kinetic activator constant" Km is a constant Km is a constant derived from rate constants Km is, under true Michaelis-Menten conditions, an estimate of the dissociation constant of E from S Small Km means tight binding; high Km means weak binding The theoretical maximal velocity Vmax is a constant Vmax is the theoretical maximal rate of the reaction - but it is NEVER achieved in reality To reach Vmax would require that ALL enzyme molecules are tightly bound with substrate Vmax is asymptotically approached as substrate is increased Combination of 0-order and 1st-order kinetics When S is low, the equation for rate is 1st order in S When S is high, the equation for rate is 0order in S The Michaelis-Menten equation describes a rectangular hyperbolic dependence of v on S! A measure of catalytic activity kcat, the turnover number, is the number of substrate molecules converted to product per enzyme molecule per unit of time, when E is saturated with substrate. If the M-M model fits, k2 = kcat Values of kcat range from less than 1/sec to many millions per sec Name for kcat/Km An estimate of "how perfect" the enzyme is kcat/Km is an apparent second-order rate constant It measures how the enzyme performs when S is low The upper limit for kcat/Km is the diffusion limit - the rate at which E and S diffuse together Catalytic perfection (rate of reaction being diffusion-controlled) can be achieved by a combination of different values of kcat and Km.