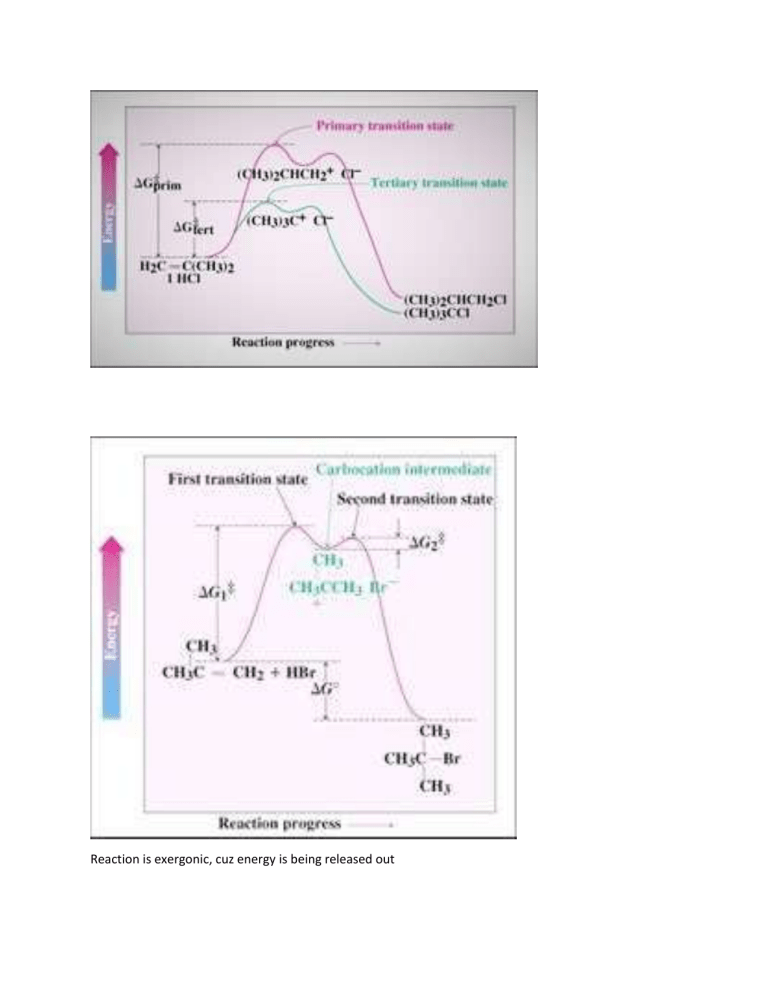
Reaction is exergonic, cuz energy is being released out Comparing Stabilities of Alkenes Evaluate heat given off when C=C is converted to C-C More stable alkene gives off less heat Trans butene generates 5 kJ less heat than cis-butene Electrophilic Addition forpreparations The reaction is successful with HCl and with HI as well asHBr Note that HI is generated from KI and phosphoric acid Addition of hydrogen bromide to 2-Methyl-propene H-Br transfers proton toC=C Forms carbocation intermediate More stable cation forms Bromide adds to carbocation 7.4 Addition of Water to Alkenes:Oxymercuration Hydration of an alkene is the addition of H-OH to to givean alcohol Acid catalysts are used in high temperature industrialprocesses: ethylene is converted to ethanol