Alkene Addition Reactions Quiz Questions
advertisement

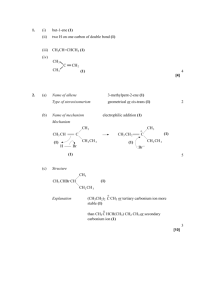
Chapter 5. Addition Reactions of Alkenes Sample Quiz Questions 5.6 Contrast the products expected when 3-methyl-1-butene undergoes (a) acid-catalyzed hydration or (b) oxymercuration-reduction. Explain any differences. A) B) Carbocation forms on 2o carbon which then shifts (hydride shift) to the next carbon making it a 3o carbon. A water molecule then bonds where the carbocation is. No carbocation is formed. Hg bonds to least substituted carbon of the double bond, leaving the more substituted carbon partially positive and able to accept electrons from a water molecule. 5.10b Provide the structure of an alkene that would give the following alcohol as the major (or only) product of hydroboration-oxidation. CH3 CH2 CH CH OH CH3 CH3 5.13-14 Give the products (if any) expected from the treatment of the 3-methyl-2-pentene with ozone followed by (a) dimethyl sulfide (reductive workup) and (b) aqueous hydrogen peroxide (oxidative workup). A) B) 5.15 In each case, give the structure of an eight-carbon alkene that would yield each of the following compounds (and no others) after treatment with ozone followed by dimethyl sulfide (reductive workup). 0 (a) O 0 O (b) CH (CH2)5 C CH3 CH3 CH2 CH2 CH O O 0 (c) O 0 5.29 Give the structure of (a) a six-carbon alkene that would give the same product from reaction with HBr whether peroxides are present or not. (b) two stereoisomeric alkenes that would give 3-hexanol as the major product of hydroboration followed by treatment with alkaline H2O2. 5.31d Give the missing reactant for the following equation. 0 Br ? HBr peroxides (only product)