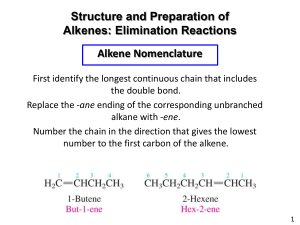
Organic Chemistry 2 Alkenes Summary Homework 1. State the general formula of an alkene 2. Define the following key terms: a. Hydrocarbon b. Unsaturated c. Stereoisomer d. Electrophile e. Addition reaction f. Polymerisation 3. Name the following compounds: a. b. c. 4. Draw the following compounds: a. 2-methylpent-2-ene b. Hexa-2,4-diene c. 3-ethyloct-3-ene 5. What is the difference between a sigma and a pi bond? Draw diagrams to show the formation of a pi bond. 6. Why do stereoisomers for but-2-ene exist but not 2-methlybut-2-ene? Draw and name the different isomers for but-2-ene. 7. Compare the boiling points of alkanes and alkenes. 8. Are alkenes soluble in water? Explain your answer. 9. Describe the different tests for identifying alkenes. Include any expected observations. 10. Draw separate mechanisms for the addition of H2, Cl2 and HCl to propene. Include reagents and conditions where necessary. 11. Draw the repeating unit of poly(propene)