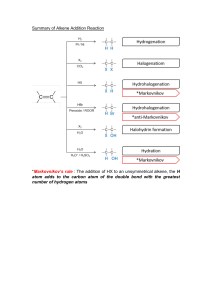
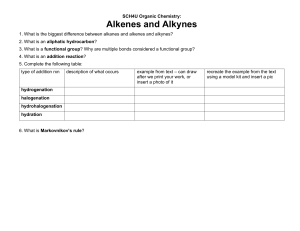
Learning Outcome : Predict the products of addition reactions of alkenes and alkynes and use addition reactions to complete multistep synthesis problems. Addition Reactions of Alkenes ______________________ Alkenes are electron rich. The electron density of the bond is concentrated above and below the plane of the molecule. Alkenes are nucleophiles; therefore, they react with electrophiles. Hydrohalogenation—Electrophilic Addition of HX ____________________ Two bonds are broken and two new bonds are formed. The H—X bond is polarized. This structural feature makes HX a good electrophile. Mechanism – Electrophilic Addition of HX to an Alkene Regiochemistry: Markovnikov’s Rule and the Addition of HX to an Alkene ______________________ The addition of HX to an unsymmetrical alkene follows Markovnikov’s rule. The rule states that in the addition of HX to an unsymmetrical alkene, the H atom bonds to the carbon that has the greater number of hydrogens (“The rich get richer.”) The Physical Basis for Markovnikov’s Rule Stereochemistry of Addition Reactions Hydrohalogenation—Reaction Stereochemistry A racemic mixture Hydrohalogenation—Reaction Stereochemistry • A and B are enantiomers. Since attack from either direction occurs with equal probability, a racemic mixture of A and B is formed. EP2: Predict the products of the following reactions: _____________________ Cyclopentene + HI 1,2-dimethylcyclohexene + HBr EP4: Draw the products formed when each alkene is treated with HBr: ______________________ 1-methylcyclohexene 2-methylpropene 2-methyl-1-hexene EP5: Draw the product of the following reaction: ______________________ 1,5,5-trimethylcyclohexene + HBr Carbocation Rearrangement: A 1,2-hydride Shift © 2014 Pearson Education, Inc. Carbocation Rearrangement: A 1,2-methyl Shift © 2014 Pearson Education, Inc. The carbocation does not rearrange when there is no improvement in the stability of the carbocation. © 2014 Pearson Education, Inc. EP6: Predict the product(s) for the following addition reactions: _____________________ HBr to 3-methyl-1-pentene ICl to 3-methylcyclohexene EP7: Provide mechanistic explanations for the following observations: ______________________ The addition of hydrogen chloride to 3methyl-1-butene produces two products: 2chloro-3-methylbutane and 2-chloro-2methylbutane. The addition of hydrogen chloride to 3,3dimethyl-1-butene produces two products: 3chloro-2,2-dimethylbutane and 2-chloro-2,3dimethylbutane. Addition Reactions of Alkenes Hydration—Electrophilic Addition of Water • Hydration is the addition of water to an alkene to form an alcohol. Mechanism - Electrophilic Addition of Water Hydration: Electrophilic Addition of Water ______________________ Three consequences to the formation of carbocation intermediates: Markovnikov’s rule applies Addition of H and OH occurs in both syn and anti fashion Carbocation rearrangements can occur EP8: What two alkenes give rise to each alcohol as the major product by acid-catalyzed hydration? 2-methyl-2-pentanol 1-methylcyclohexanol 2-butanol Hydration: Oxymercuration - Demercuration ______________________ Reaction is initiated by electrophilic addition of Hg2+ ion to the alkene Regiochemistry of the reaction corresponds to Markovnikov addition of H2O Anti Addition Hydroboration—Oxidation The net result is the addition of H2O to an alkene. Hydroboration—Oxidation With unsymmetrical alkenes, the boron atom bonds to the less substituted carbon atom. The larger boron atom bonds to the less sterically hindered, more accessible carbon atom. Hydroboration—Oxidation The overall result of this two-step sequence is a syn anti-Markovnikov addition of the elements of H and OH to a double bond. EP5: Draw the products formed when each alkene is treated with BH3 followed by H2O2 and OH-. 1-butene 1-ethylcyclopentene 1,4-dimethylcyclohexene EP6: Acid-Catalyzed Hydration versus HydroborationOxidation Draw the product formed in each reaction Methylenecyclopentane + H2O/H2SO4 Methylenecyclopentane + (1) BH3 + (2) H2O2, OH- Learning Outcome: Predict the regio- and stereochemistry of addition reactions of alkenes. Halogenation—Addition of Halogen • Halogenation is the addition of X2 (X = Cl or Br) to an alkene to form a vicinal dihalide. Halogenation—Addition of Halogen Mechanism for the Addition of X2 to an Alkene There are two observations that suggest that the addition of X2 follows a mechanism that is different from that of the addition of HX and H2O: There are no rearrangements of the carbon skeleton. Only anti addition occurs. Mechanism for the halogenation reaction of alkenes: Halogenation—Reaction Stereochemistry Halogenation – Reaction Stereochemistry EP1: Provide a step-by-step mechanism for the halogenation reaction involving cyclobutene with Br2. EP2: Draw the products of each reaction, including stereochemistry. 4-methylcyclopentene + Br2 1-methylcyclohexene + Cl2 Halohydrin Formation – Addition of X and OH Halohydrin Formation Even through X¯ is formed in step [1] of the mechanism, its concentration is small compared to H2O. Halohydrin Formation Backside attack of H2O leads to X and OH being added in an anti fashion and the formation of trans products. With unsymmetrical alkenes, the major product is the one in which the electrophile X+ is bonded to the less substituted carbon, and the nucleophile (H2O) binds to the more substituted carbon. Halohydrin Formation Nucleophilic attack occurs at the more substituted carbon because that carbon is better able to accommodate the partial positive charge in the transition state. EP3: Draw the products of each reaction, including stereoisomers: Cyclobutene + Cl2 in H2O methylenecyclopentane + Br2 in H2O