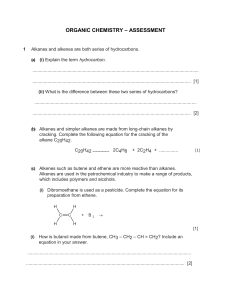
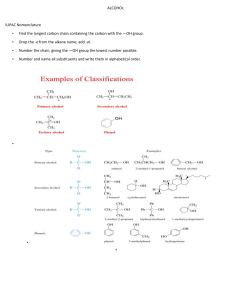
Alkanes and Alkenes Worksheet How can we distinguish between alkanes and alkenes? Aim: To work out the best way to distinguish alkanes from alkenes. 1 Given below are the structures of four hydrocarbons: A B C D Explain why all four compounds above are hydrocarbons. .......................................................................................................................................................... .......................................................................................................................................................... 2 Classify the compounds A to D as either alkanes or alkenes. Compound Molecular formula A B C D 3 The general formula for alkanes is CnH2n+2. What is the general formula for alkenes? ………………………. 4 Classify the following compounds as either alkanes or alkenes: E C3 H8 alkane/alkene F C5H10 alkane/alkene G C6H14 alkane/alkene H C6H12 alkane/alkene I C5H12 alkane/alkene Alkane or alkene? 5 Distinguishing between alkanes and alkenes using chemical reactions: Test alkane alkene Combustion Bromine water 6 Which of these tests is the better one? Explain your answer. ....................................................................................................................... 7 Complete the following true/false statements about alkenes. a) All alkenes are hydrocarbons. true/false Reason .................................................................................................................................... ................................................................................................................................................. b) Their general formula is CnH2n+2. true/false Reason .................................................................................................................................... ................................................................................................................................................. c) Ethene is an alkene. true/false Reason .................................................................................................................................... d) Alkenes decolourise bromine water. true/false Reason .................................................................................................................................... e) C4H10 is an alkene. true/false Reason .................................................................................................................................... f) Alkenes burn with a non-smoky flame. true/false Reason .................................................................................................................................... ................................................................................................................................................. g) The formula C4H8 represents an alkene. true/false Reason .................................................................................................................................... .................................................................................................................................................