Elements and Compounds Element – A fundamental substance that
advertisement

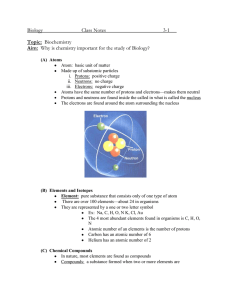
Elements and Compounds Element – A fundamental substance that cannot be broken down by simple chemical and physical processes. This substance is made of only one kind of atom, such as oxygen (O₂). There are 90 naturally occurring elements on earth. Compound – A substance made of two or more different kinds of atoms. Carbon dioxide (CO₂), sugar (C₆H₁₂O₆), and water (H₂O) are compounds. Oxygen (O₂) and hydrogen (H₂) are not compounds. (They are elements, as they are made of only one kind of atom). **Atoms are made out of three main smaller parts. They are protons, neutrons, and electrons. Periodic Table – a way to organize the elements based on atomic number. The atomic number is the number of protons in the nucleus of the element’s atom. Color also shows which elements have similar chemical properties. For example, all nonmetals are shown in yellow. Nucleus – the center of an atom. Protons – a subatomic particle that has a positive charge. The number of protons in an atoms’s nucleus dictates its place on the Periodic Table. For example, hydrogen only has one proton in its nucleus, therefore, it is the first element represented on the Periodic Table. Neutrons – A subatomic particle that with no charge. Electrons – A subatomic particle with a negative charge. **What makes atoms different from each other is the number of protons, neutrons and electrons each has.