PROPERTIES OF MATTER
advertisement

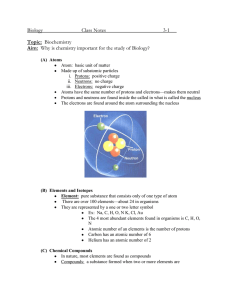
ATOM PROPERTIES OF MATTER PROPERTIES ELEMENTS COMPOUNDS & MIXTURES *They are called the “building blocks of matter”. *All Matter has both chemical and physical properties *Made up of electrons neutrons and protons *Chemical properties can change matter to a new kind of matter with different properties. An example is burning paper to ash. Modeling clay is malleable. It can be changed buts its make-up or identity remains unchanged, Therefore this is a physical change NOT chemical. *Electrons have negative charges. They are located outside the nucleus. *Neutrons are found in the nucleus. They hold no charge *Protons are also found in the nucleus. They have a positive charge. There are about 110 elements and 92 are natural while the rest are synthetic or man made. All Matter in the universe is made of elements or compounds. An element is the simplest form of matter. It can’t be made any simpler and only contains one kind of atom. Oxygen is made out of only oxygen (O2) atoms. Therefore it is considered pure. Carbon is another example. Two or more atoms combined is called a molecule. Compounds contain 2 or more elements in order to form a compound. Most elements are found in combination. With other elements. Table salt is an example of a compound (NaClsodium and chlorine) Mixtures are combinations of two or more substances. They can contain elements, compounds or both in any amounts. Because the substances are not chemically combined in a mixture they can be separated by physical means. Fruit salad is an example. Solutions are mixtures that look like a single substance like when salt dissolves (spreads out evenly) in boiling water. Within a solution, one substance is dissolved in another substance.