Science 8 Example Items for ACP Preparation
advertisement

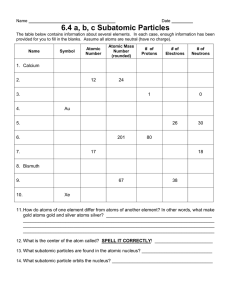
Example Items Science 8 Science 8 Example Items are a representative set of items for the ACP. Teachers may use this set of items along with the test blueprint as guides to prepare students for the ACP. On the last page, the correct answer, content SE and SE justification are listed for each item. The specific part of an SE that an Example Item measures is NOT necessarily the only part of the SE that is assessed on the ACP. None of these Example Items will appear on the ACP. Teachers may provide feedback regarding Example Items. (1) Download the Example Feedback Form and email it. The form is located on the homepage of Assessment.dallasisd.org. OR (2) To submit directly, click “Example Feedback” after you login to the Assessment website. First Semester 2017–2018 Code #: 3081 STAAR GRADE 8 SCIENCE REFERENCE MATERIALS STAAR TM State of Texas Assessments of Academic Readiness FORMULAS Density = mass volume Average speed = total distance total time Net force = (mass)(acceleration) Work = (force)(distance) D = m V s = d t F = ma W = Fd 7 6 5 4 3 2 1 4 Hydrogen 3 12 11 (262) (267) 7 7B Actinide Series Lanthanide Series 140.908 231.036 Protactinium 232.038 Thorium (227) Actinium 91 Pa Ac Re 144.242 60 Nd Bohrium (272) Bh 107 Rhenium 186.207 75 (145) 61 Pm Hassium (270) Hs 108 Osmium Os 190.23 76 Ruthenium 101.07 44 Ru Iron 55.845 Fe 26 8 Silicon 28.086 Si Uranium 238.029 U 92 Neptunium (237) 93 Np Praseodymium Neodymium Promethium Th 90 Cerium Lanthanum 89 140.116 La 138.905 59 58 Pr (271) Seaborgium (268) Sg Dubnium Db Tungsten Tantalum 106 W 183.84 74 Ta 105 (98) 43 Tc Manganese 54.938 Mn 25 Molybdenum Technetium 95.96 42 Mo Chromium 51.996 Cr 24 6 6B Atomic mass Symbol 180.948 73 Niobium 92.906 41 Nb Vanadium 50.942 V 23 5 5B Ce 57 Lawrencium Rutherfordium (226) Radium 104 Rf Hafnium 103 (223) Fr Hf 178.49 Lutetium Francium 88 87 Lr Barium Cesium 174.967 Lu 72 71 Ra Ba 137.328 Cs 132.905 56 55 Zirconium 91.224 40 Zr Yttrium 88.906 87.62 Strontium 85.468 Rubidium Y 39 Sr 38 Rb 37 47.867 Titanium 44.956 Scandium 40.078 Calcium 39.098 Potassium 22 Ti 21 4 4B Sc 19 3 3B Ca 20 Sodium K 24.305 Magnesium 22.990 Mg Beryllium Lithium Na Be 9.012 Li 6.941 1.008 2 2A H Name 10 11 1B 12 2B 13 3A 14 4A 15 5A 16 6A 17 7A (281) Ds 110 Platinum Pt 195.085 78 Palladium 106.42 46 Pd Nickel 58.693 Ni 28 Au (280) Rg 111 Gold 196.967 79 Silver 107.868 47 Ag Copper 63.546 Cu 29 Plutonium (244) 94 Pu Samarium 150.36 62 Sm (247) Curium (243) 96 Cm Americium 95 Am 157.25 Gadolinium 151.964 64 Gd Europium 63 Eu Meitnerium Darmstadtium Roentgenium (276) Mt 109 Iridium Ir 192.217 77 Rhodium 102.906 45 Rh Cobalt 58.933 Co 27 9 8B Hg Tl Thallium 204.383 81 Indium 114.818 49 In Gallium 69.723 Ga 31 Aluminum 26.982 Al 13 Boron C Pb Lead 207.2 82 Tin 118.711 50 Sn Germanium 72.64 Ge 32 Silicon 28.086 Si 14 Carbon 12.011 6 N Bi Bismuth 208.980 83 Antimony 121.760 51 Sb Arsenic 74.922 As 33 30.974 Phosphorus P 15 Nitrogen 14.007 7 Berkelium (247) 97 Bk Terbium 158.925 65 Tb Californium (251) 98 Cf Dysprosium 162.500 66 Dy Einsteinium (252) 99 Es Holmium 164.930 67 Ho Fermium (257) 100 Fm Erbium 167.259 68 Er Mass numbers in parentheses are those of the most stable or most common isotope. Mercury 200.59 80 Cadmium 112.412 48 Cd Zinc 65.38 Zn 30 B 10.812 5 O Po F At Nobelium (259) 102 No Ytterbium 173.055 70 Yb Astatine (210) 85 Iodine 126.904 I 53 Bromine 79.904 Br 35 Chlorine 35.453 Cl 17 Fluorine 18.998 9 Updated Spring 2011 Mendelevium (258) 101 Md Thulium 168.934 69 Tm Polonium (209) 84 Tellurium 127.60 52 Te Selenium 78.96 Se 34 Sulfur 32.066 S 16 Oxygen 15.999 8 Ne Rn Radon (222) 86 Xenon 131.294 54 Xe Krypton 83.798 36 Kr Argon 39.948 18 Ar Neon 20.180 10 Helium 4.003 He 2 14 1 Atomic number 18 8A 1 1A PERIODIC TABLE OF THE ELEMENTS STAAR GRADE 8 SCIENCE REFERENCE MATERIALS EXAMPLE ITEMS Science 8, Sem 1 1 2 3 The reaction NH3 O2 NO H2O is considered unbalanced because there are — A more oxygen atoms on the reactant side of the equation than on the product side of the equation B less nitrogen atoms on the reactant side of the equation than on the product side of the equation C more hydrogen atoms on the reactant side of the equation than on the product side of the equation D unequal numbers of all atoms on the reactant and product sides of the equation When George flies his model airplane, he wants to know how fast and the direction it is flying. What does he calculate to tell him both? A Acceleration B Distance C Speed D Velocity The properties of an unknown element are listed. Reactivity Electrical Conductivity Malleability Very High Very Good Good Based on these properties, the element is expected to be listed in which group on the Periodic Table? A 1 B 16 C 17 D 18 Dallas ISD - Example Items EXAMPLE ITEMS Science 8, Sem 1 Use the picture to answer the next question. 4 5 How many protons are present in the oxygen atom? A 24 B 16 C 8 D 4 In a crash test demonstration, a car with a bowling ball inside hits a brick wall. The bowling ball then flies out of the car because it is not restrained. Which statement best describes why this happens? A An object will remain at rest or move in a straight line unless it is acted upon by another force. B An object acted upon by an unbalanced force will accelerate in the direction of the force. C An object will resist motion if the surfaces of the two objects resist each other. D For every action, there is an equal but opposite reaction. Dallas ISD - Example Items EXAMPLE ITEMS Science 8, Sem 1 6 Which table matches the subatomic particles with the region of an atom in which they are located? A B C D 7 Subatomic Particles Location in Atom Protons and Electrons Nucleus Neutrons Surrounding nucleus Subatomic Particles Location in Atom Electrons and Neutrons Nucleus Protons Surrounding nucleus Subatomic Particles Location in Atom Protons and Neutrons Nucleus Electrons Surrounding nucleus Subatomic Particles Location in Atom Electrons Nucleus Protons and Neutrons Surrounding nucleus The picture shows a divergent boundary between two continental landmasses. Faults Tectonic plates moving away from each other Mantle Continental Crust What crustal formation results from this motion? A Folded mountain B Rift valley C Trench D Surface volcano Dallas ISD - Example Items Magma EXAMPLE ITEMS Science 8, Sem 1 Use the table to answer the next question. 8 9 Common Name Chemical Formula Hydrogen peroxide 2H2O2 Vinegar CH3CO2H Glucose C6H12O6 Iron oxide 3 Fe2O3 Which chemical has nine atoms of oxygen? A Hydrogen peroxide B Vinegar C Glucose D Iron oxide Students added white tablets to the cup of water. Before After Which statement indicates that a chemical change occurred? A Gas bubbles formed. B The tablets dissolved. C The density of the tablets decreased. D The volume of the water decreased. Dallas ISD - Example Items EXAMPLE ITEMS Science 8, Sem 1 10 11 Which statement provides the best evidence that the Earth’s continents are in very different positions today than they were millions of years ago? A Glaciers form valleys between mountains. B Volcanoes surround most of the Pacific Ocean. C Polar bears have become an endangered species. D Fossils of tropical plants have been found in Antarctica. Students were given the topographic map. An approximate elevation for Point Z is — A 100 m B 200 m C 220 m D 300 m Dallas ISD - Example Items EXAMPLE ITEMS Science 8, Sem 1 Use the diagram to answer the next question. 12 What is the net force in newtons on the cylinder? Record the answer and fill in the bubbles on the grid provided. Be sure to use the correct place value. 13 Which scenario includes a description of velocity? A Two skateboarders racing on a fifteen meter ramp B Two skateboarders traveling north at the same speed C Two skateboarders traveling at a constant speed D Two skateboarders waiting at a starting line Dallas ISD - Example Items EXAMPLE ITEMS Science 8, Sem 1 Use the table to answer the next question. Chemical Equations 14 15 I 3Fe H2O Fe3O4 H2 II C2H6 3O2 2CO2 3H2O III 2Zn SO2 2ZnO S Which equations do not follow the law of conservation of mass? A I and II B I, II, and III C I and III D II and III A man pulled a cart filled with stones that had a total mass of 60kg. He increased the amount of force used to pull the cart from 60N to 90N, what is the new acceleration of the cart? A 2.5 m/s2 B 1.5 m/s2 C 1 m/s2 D 0.5 m/s2 Dallas ISD - Example Items EXAMPLE ITEMS Science 8 Key, Sem 1 Item# Key SE Process Skills SE Justification 1 C 8.5F -- Recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of mass. 2 D 8.6B -- Differentiate between speed, velocity, and acceleration. 3 A 8.5C 8.2E Interpret the arrangement of the Periodic Table, including groups, to explain how properties are used to classify elements. 4 C 8.5B 8.3B Identify that protons determine an element's identity and valence electrons determine its chemical properties, including reactivity. 5 A 8.6C -- Investigate and describe applications of Newton's law of inertia, law of force and acceleration, and law of action reaction such as in vehicle restraints. Describe the structure of atoms, including the masses, electrical charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud. 6 C 8.5A -- 7 B 8.9B 8.3B Relate plate tectonics to the formation of crustal features. Recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts. 8 D 8.5D 8.2E 9 A 8.5E -- Investigate how evidence of chemical reactions indicate that new substances with different properties are formed. 10 D 8.9A -- Describe the historical development of evidence that supports plate tectonic theory. 11 C 8.9C 8.3B 12 5 8.6A -- Calculate how unbalanced forces change the direction of an object's motion. 13 B 8.6B -- Differentiate between velocity and acceleration 14 A 8.5F -- Recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of mass 15 B 8.6A -- Demonstrate and calculate how unbalanced forces change the speed of an object's motion Interpret topographic maps and satellite views to identify land features.