Biology Class Notes 3-1
advertisement
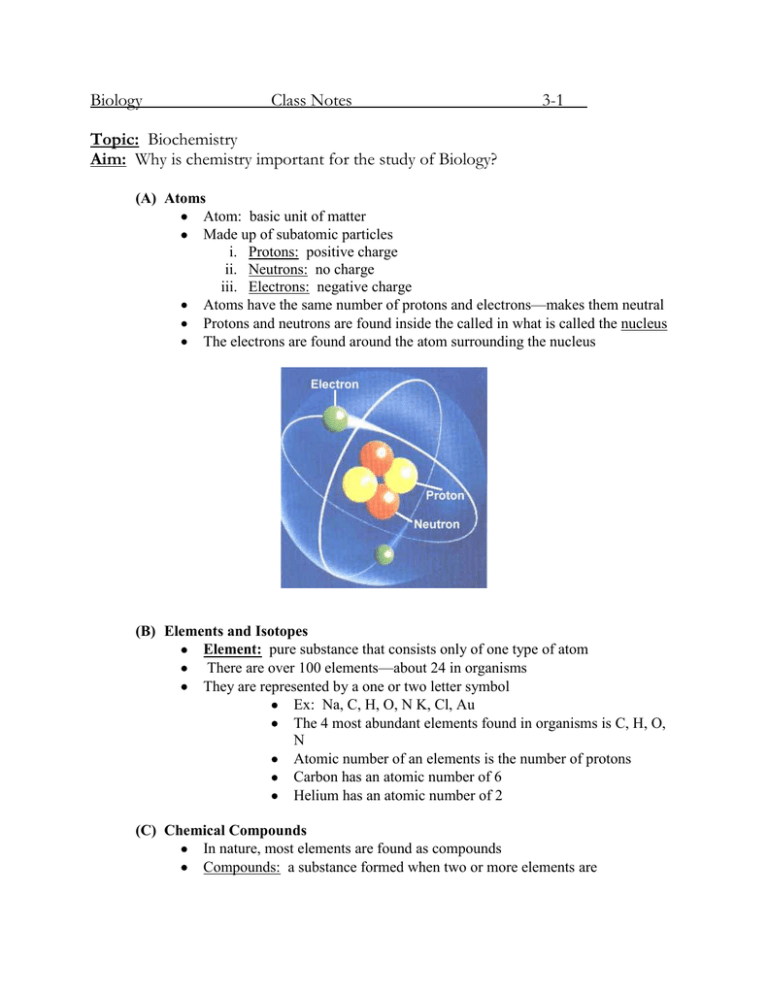
Biology Class Notes 3-1 Topic: Biochemistry Aim: Why is chemistry important for the study of Biology? (A) Atoms Atom: basic unit of matter Made up of subatomic particles i. Protons: positive charge ii. Neutrons: no charge iii. Electrons: negative charge Atoms have the same number of protons and electrons—makes them neutral Protons and neutrons are found inside the called in what is called the nucleus The electrons are found around the atom surrounding the nucleus (B) Elements and Isotopes Element: pure substance that consists only of one type of atom There are over 100 elements—about 24 in organisms They are represented by a one or two letter symbol Ex: Na, C, H, O, N K, Cl, Au The 4 most abundant elements found in organisms is C, H, O, N Atomic number of an elements is the number of protons Carbon has an atomic number of 6 Helium has an atomic number of 2 (C) Chemical Compounds In nature, most elements are found as compounds Compounds: a substance formed when two or more elements are The physical and chemical properties of a compound are different from the elements from which it is formed H=gas O=gas water Some compounds are carbon dioxide CO2, water H2O, sodium chloride NaCl, glucose C6 H12O 6 Compounds are formed through chemical bonding Ionic: one electron is given to another atom Covalent: electrons are shared