Understanding Atomic Structure using a Periodic Table Periodic Table
advertisement

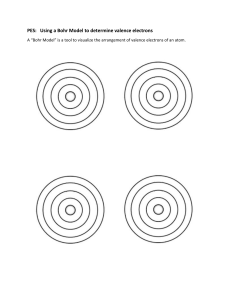
Name: ____________________________________________ Block: _________ Date: __________ Understanding Atomic Structure using a Periodic Table Periodic Table Using the periodic table given to you complete the following chart Element (ATOM) Sodium Atomic Symbol Atomic Number Atomic Mass (round) Proton # Neutron # Electron # Beryllium Nitrogen Helium Iodine Silver Bohr Models Practice drawing Bohr Models for the following atoms (make sure to note the proton, neutron, electron and valence number) Li F Na Ar Complete the Chart Fill in the following chart using your periodic table and what you have learned about groups and periods Element Period in which the element is located Number of Energy Levels Group in which the element is located Number of Valence Electrons Sodium (Na) Lithium (Li) Carbon (C) Silicon (Si) Argon (Ar) Covalent Bonding: Show the sharing of electrons (using a Bohr model) to create a covalent bond between the following atoms. 1. Hydrogen Atom + Hydrogen Atom = Hydrogen Molecule (H2) Ionic Bonding: Show the transfer of electrons to create an ionic bond using Bohr models. 2. Magnesium Chloride (MgCl2)