SPECTRA OF SCIENCE Chapter 4 Learning Targets
advertisement

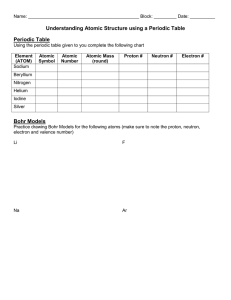
SPECTRA OF SCIENCE Chapter 4 Learning Targets Directions: Use the rating scale below to determine how well you know and can perform each of the learning targets below. Rating Scale: 0 What’s that? 1 I know a little bit 2 I know a lot # Learning Targets: 1 I can define and differentiate between atoms, isotopes, cations, and anions, and discuss how each is formed. 2 I can identify the 3 subatomic particles, and describe them in terms of location and charge. 3 I am able to construct accurate Bohr models and Lewis Dot diagrams of atoms. 4 I can determine an ion’s charge by evaluating the number of protons and electrons it contains. 5 I can use the periodic table to identify the number of protons, neutrons, and electrons in an atom. 6 I can use the periodic table to identify key information about elements including atomic number, atomic mass, elemental symbol, and most commonly occurring ions. 7 I am able to discuss relative milestones and the scientists who contributed in the development of atomic theory. 8 I can explain the organization of the periodic table and identify similar properties of elements given their location in the table. 3 I could teach this! Before After SPECTRA OF SCIENCE Chapter 4 Study Guide **Due on: _______________________** Directions: Complete each of the items below on a separate sheet of notebook paper. Be sure to number each item. 1. List the three subatomic particles. Then describe their charge and their location. 2. How do electrons are believed to behave in the most modern models of the atom? 3. Draw Bohr models for the elements below: a. Carbon b. Argon c. Aluminum 4. Determine the charge of the ions listed below. a. An Oxygen atom with 10 electrons b. An Fluorine atom with 10 electrons c. A Beryllium atom with 2 electrons 5. Complete the chart below. Element Atomic Atomic # of # of # of Symbol Name Number Mass Protons Neutrons Electrons Manganese Cs 33 79 26 6. Identify the contributions to atomic theory made by each of the scientists listed below: a. Democritus b. John Dalton c. Niels Bohr 7. Identify the location and describe the characteristic properties of each of the elemental families or groups listed below: a. Noble gases d. Alkaline Earth metals b. Halogens e. Metals c. Alkali Metals f. Nonmetals 8. Identify the most likely ion formed by the following groups of elements listed below: a. Groups 1 c. Group 17 b. Group 2 d. Group 18 9. How is the periodic table organized? 10. Define the Vocabulary words listed below: a. Nucleus f. Anion b. Ionization g. Valence electron c. Ion h. Orbitals d. Isotope i. Semi-conductor e. Cation j. Amu