Chemistry Test Content
advertisement

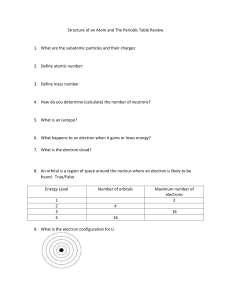
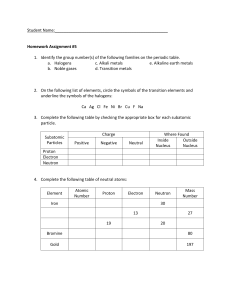
CHEMISTRY TEST CONTENT STUDY SMART, NOT HARD: KNOW THE FOLLOWING FOR OUR UNIT ASSESSMENT Part One: Periodic Table Basics Descriptions of solids, liquids, gases Subatomic particles, charges, and locations o Protons (+) o Neutrons (o) o Electrons (-) Electron energy shells Valence electrons (How do you determine the number of valence electrons?) Making Electron Diagrams/Bohr Models Groups/Periods Alkali metals, Alkaline Earth metals, Transition metals, Metalloids, Halogens, Noble Gases Atomic Number Atomic Mass How reactivity changes throughout the Periodic Table How atomic mass changes throughout the Periodic Table Part Two: Chemical Changes Common Compounds Specific Elements and their symbols How to tell if a Chemical or Physical Reaction has taken place Coefficients Subscripts Conservation of Mass (How do you know if mass is conserved? What does it mean?)