Chemistry Semester 1 Final Exam Topics
advertisement

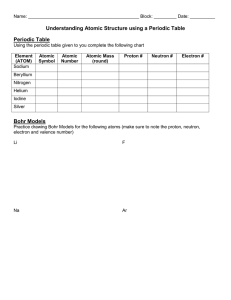
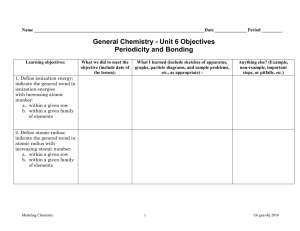
Chemistry Semester 1 Final Exam Topics You will be able to use one 3x5 note card during the exam. A Periodic Table and Nomenclature Data Sheet will be supplied during the exam. Materials to bring: #2 pencils and eraser calculator Topics: Chapter 1: Introduction to Chemistry o Key Terms: chemistry, scientific method, hypothesis, theory, natural law Chapter 2: Matter and Change o Key Terms: matter, atom, element, compound, mixture (homogeneous and heterogeneous), filtration, distillation o Be able to classify matter as an element, compound, mixture, etc. o Know and understand the three states of matter (s,l,g) o Be able to identify a chemical and a physical property and change Chapter 3: Scientific Measurement o Be able to write numbers in scientific notation and standard form o Know the major units of measurement o Be able to identify the number of significant figures in a measurement o Be able to perform calculations using scientific notation and significant figures o Be able to correctly round a number o Be able to convert between units (dimensional analysis) o Be able to convert between the Celsius and Kelvin temperature scales o Understand and be able to solve density problems Chapter 4: Atomic Structure o Be able to identify elements on the periodic table o Know the basic structure of an atom o Be able to find the number of protons, electrons, neutrons, atomic number and mass number for a neutral atom, ion, and isotope o Know the diatomic gases Chapter 5: Electrons in Atoms o Key Terms: wavelength, frequency, electromagnetic radiation, ground state, excited state, quantized o Be able to calculate wavelength, frequency, and energy of electromagnetic radiation o Know how to determine the number of valence electrons o Know how to recognize the correct electron configuration for an element Chapter 6: The Periodic Table o Be able to identify an element as a metal, nonmetal or metalloid o Be able to identify an element as an alkali metal, alkaline earth metal, transition metal, halogen, or noble gas o Know trends of the periodic table for atomic size, ionization energy and electronegativity. Chapter 7: Ionic Bonding and Chapter 8: Covalent Bonding o Know how to determine the number of valence electrons for an element o Know how cations and anions are formed o Know the difference between an ionic, covalent and polar covalent bond o Be able to determine bond type o Be able to predict the formula for a compound o Be able to draw Lewis Structures o Be able to determine molecular geometry Chapter 9: Chemical Names and Formulas o Be able to name or write formulas for compounds (ionic, covalent, acids, hydrates Chapter 10: Chemical Quantities o Be able to calculate molar mass o Know and understand what a mole is (Avogadro’s Number) o Be able to convert between grams, moles, or atoms/molecules of a substance Chapter 11: Chemical Reactions o Be able to identify the reactants and products in a chemical equation o Be able to balance a chemical equation o Understand the difference between a coefficient and a subscript o Be able to determine reaction type (Synthesis, Decomposition, Single Replacement, Double Replacement, Acid Base, Oxidation-Reduction, Combustion)