ELECTRONEGATIVITY
advertisement

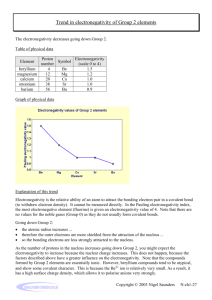
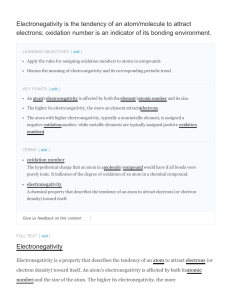
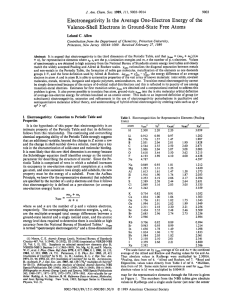
ELECTRONEGATIVITY Electronegativity is a concept which is used to help understand which atom in a chemical bond will have the greatest attraction for the bonding pair of electrons. Electronegativity is a measure of the ability of an atom in a chemical bond to attract electrons to itself. From the figure one can see the important trend in electronegativity across a period and down a group. In general the electronegativity increases going across a period, and decreases going down a group.