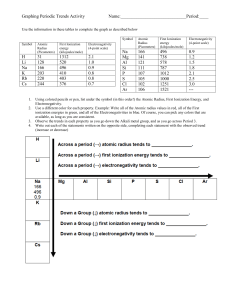
Periodic Trends Worksheet: Atomic Radius, Ionization Energy
advertisement

Periodic Trends Worksheet 1. What trend do you see in atomic radius as you go down a group? 2. What causes this trend? 3. Circle the atom in each pair that has the largest atomic radius a. Al or B b. S or O d. Na or Al c. Br or e. O or F Cl f. Mg or Ca 4. Define ionization energy 5. Why does sodium form a 1+ ion while magnesium forms a 2+ ion? 6. Circle the atom in each pair that has the greater ionization energy. a. Li or Be b. Na or K c. Cl or Si d. Ca or Ba e. P or Ar f. Li or K 7. Define electronegativity. What trend in electronegativity do you see moving across a period? 8. Circle the atom in each pair that has the greater electronegativity. a. Ca or Ga b. Li or O c. Cl or S d. Br or As e. Ba or Sr f. O or S 9. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. 10. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum. 11. What is the difference between electronegativity and ionization energy? 12. Why does fluorine have higher ionization energy than iodine?