Bond Polarity 2018
advertisement

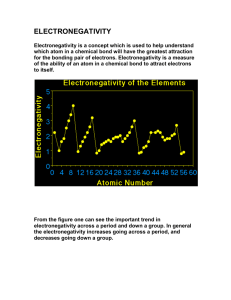
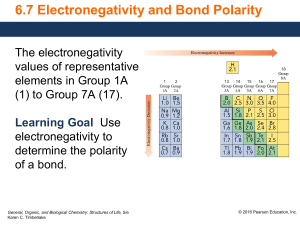
Bond Polarity Most bonds are a blend of ionic and covalent characteristics. Difference in electronegativity determines bond type. Electronegativity is a measurement of the tendency of an atom to attract a bonding pair of electrons. increases from left to right going across a period on the periodic table. is high for the nonmetals with fluorine as the highest. is low for the metals. When you subtract the electronegativity it’s a positive number. If you subtract them and its negative switch to positive! A nonpolar covalent bond is covalent bonds that share electrons equally. Generally, there is a 0.0-0.4 difference in the atoms’ electronegativity values. A polar covalent bond is a covalent bond that do NOT share electrons equally. Generally, there is a 0.4-1.7 difference in the atoms’ electronegativity values. Ionic Bond is between a metal and non-metal that has a complete electron transfer (1.8 or higher Electronegativity difference). Notation for Polar Covalent higher e-neg atom (HIGHER NUMBER) ____________ lower e-neg atom (SMALLER Number) _____________ Draw a fancy arrow from small to big _______________