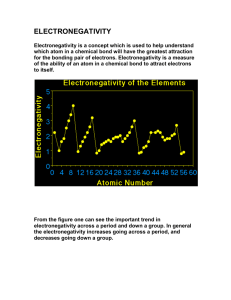
Electronegativity is the tendency of an atom/molecule to attract
advertisement

Electronegativity is the tendency of an atom/molecule to attract electrons; oxidation number is an indicator of its bonding environment. LEARNING OBJECTIVES [ edit ] Apply the rules for assigning oxidation numbers to atoms in compounds Discuss the meaning of electronegativity and its corresponding periodic trend KEY POINTS [ edit ] An atom's electronegativity is affected by both the element'satomic number and its size. The higher its electronegativity, the more an element attractselectrons. The atom with higher electronegativity, typically a nonmetallic element, is assigned a negative oxidationnumber, while metallic elements are typically assigned positive oxidation numbers. TERMS [ edit ] oxidation number The hypothetical charge that an atom in amolecule/compound would have if all bonds were purely ionic. It indicates of the degree of oxidation of an atom in a chemical compound. electronegativity A chemical property that describes the tendency of an atom to attract electrons (or electron density) toward itself. Give us feedback on this content: FULL TEXT [ edit ] Electronegativity Electronegativity is a property that describes the tendency of an atom to attract electrons (or electron density) toward itself. An atom's electronegativity is affected by both itsatomic number and the size of the atom. The higher its electronegativity, the more an element attracts electrons. The opposite of electronegativity is electropositivity, which is a measure of an element's ability to donate electrons. Electronegativity is not directly measured, but is instead calculated based on experimental measurements of other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the calculated electronegativity values, all methods show the same periodic trend among the elements. Electronegativity, as it is usually calculated, is not strictly a property of an atom, but rather a property of an atom in amolecule. Properties of a free atom include ionization energyand electron affinity. It is expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property; that is to say, similar values will be valid in a variety of situations. On the most basic level, electronegativity is determined by factors such as the nuclear charge and the number/location of other electrons present in the atomic shells. The nuclear charge is important because the more protons an atom has, the more "pull" it will have on negative electrons. Where electrons are in space is a contributing factor because the more electrons an atom has, the farther from the nucleus thevalence electrons will be, and as a result they will experience less positive charge; this is due to their increased distance from the nucleus, and because the other electrons in the lower­energy core orbitals will act to shield the valence electrons from the positively charged nucleus. The most commonly used method of calculation for electronegativity was proposed by Linus Pauling. This method yields a dimensionless quantity, commonly referred to as the Pauling scale, with a range from 0.7 to 4. If we look at the periodic table without the inert gases, electronegativity is greatest in the upper right and lowest at the bottom left. Electronegativity of the elements Electronegativity is highest at the top right of the table and lowest at the bottom left. Hence, fluorine (F) is the most electronegative of the elements, while francium (Fr) is the least electronegative. Oxidation Numbers It is common to consider a single value of electronegativity to be valid for most bonding situations a given atom can be in. While this approach has the advantage of simplicity, it is clear that the electronegativity of an element is not an invariable atomic property; rather, it can be thought of as depending on a quantity called 'the oxidation number' of the element. One way to characterize atoms in a molecule and keep track of electrons is by assigning oxidation numbers. The oxidation number is the electric charge an atom would have if the bonding electrons were assigned exclusively to the more electronegative atom, and it can identify which atom is oxidized and which is reduced in a chemical process. Six rules can be used when assigning oxidation numbers: 1. The oxidation number of an element in its natural state (i.e., how it is found in nature) is zero. For example, hydrogen in H2, oxygen in O2, nitrogen in N2, carbon indiamond, etc., have oxidation numbers of zero. 2. In ionic compounds, the ionic charge of an atom is its oxidation number. 3. The sum of the oxidation numbers of all the atoms in anion or molecule is equal to its net charge. 4. In compounds with nonmetals, the oxidation number of hydrogen is +1. However, when hydrogen is bonded with a metal, its oxidation number reduces to ­1 because the metal is a more electropositive, or less electronegative, element. 5. Oxygen is assigned an oxidation number of ­2 in most compounds. However, there are certain exceptions. In peroxides (O22­), such as hydrogen peroxide (H2O2), the oxidation number of oxygen is ­1. In oxygen difluoride (OF2), the oxidation number of oxygen is +2, while in dioxygen difluoride (O2F2), oxygen is assigned an oxidation number of +1 because fluorine is the more electronegative element in these compounds, so it is assigned an oxidation number of ­1. 6. The atom with higher electronegativity, typically a nonmetallic element, is assigned a negative oxidation number, while the other atom, which is often but not necessarily a metallic element, is given a positive oxidation number.