1) BY DEFINITION A NEUTRON HAS A CHARGE OF 0. #3
advertisement

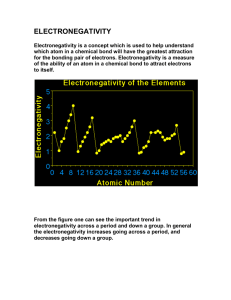
1) BY DEFINITION A NEUTRON HAS A CHARGE OF 0. #3 2) A BETA PARTICLE (A N ELECTRON OF NUCLEAR ORIGIN) IS THE SMALLEST, 1/1832 THE MASS OF A PROTON. #2 3) Cu HAS AN ATOMIC NUMBER OF 29, 29 PROTONS, WHICH IS SPECIFIC TO Cu. #1 4) AN ORBITAL IS THE MOST LIKELY LOCATION OF AN ELECTRON. #1 5) THE ELEMENTS OF THE PERIODIC TABLE ARE ARRANGED IN ORDER OF INCREASING ATOMIC #. #1 6) ACCORDING TO TABLE “S”, RHENIUM HAS THE HIGHEST MELTING POINT. #2 7) ALL CHEMICAL REACTIONS MUST BALANCE FOR MASS, CHARGE AND ENERGY. 8) DIAMOND AND GRAPHITE ARE COMPOSED OF CARBON YET HAVE DIFFERENT STRUCTUURES AND THEREFORE DIFFERENT PROPERTIES. #4 9) THE GFW (MOLAR MASS ) IS GIVEN AS 48 GRAMS/MOLE, THEREFORE 1 MOLE OF THE SUBSTANCE IS 48 GRAMS. #3 10) A CHLORINE MOLECULE ABSORBES ENERGY (BOND DISSOCIATION E) A ND BECOME 2 CHLORINE ATOMS. A) Cl2 IS A MOLECULE IN WHICH THE 2 ATOMS ARE BONDED…(THE SUBSCRIPT INDICATES THIS). B) YOU MUST ADD ENERGY TO BREAK A BOND (ENDOTHERMIC, HEAT ABSORBED). FYI-ENERGY IS RELEASED AS A STABLE BOND FORMS (BOND ENERGY) C) THEREFORE A BOND IS BROKEN AND ENERGY IS ABSORBED #1 + 11) THE MEASURE AF AN ATOMS TENDENCY TO ATTRACT AND HPOLD ELECTRONS IS ELECTRONEGATIVITY, HIGH ELECTRONEGATIVITY IS INDICATIVE OF ATOMS THAT GAIN ELECTRONS. A) THE WEAKEST ELECTRONEGATIVITY IS FOUND IN ELEMENTS ON THE LOWER LEFT . B) YOU CAN ALSO ASSESS ELECTRONEGATIVITY FROM LOOKING UP THE NUMBER ON TABLE S, HIGHER NUMBERS : MORE LIKELY TO GAIN. C) ELECTRONEGATIVITY DECREASES AS YOU READ DOWN ANY GROUP. D) S IS THE MOST BOTTOM LEFT OF ALL OF THE CHOICES, AND HAS THA LOWEST ELECTRONEGATIVITY FROM THABLE S. #4 13) THE SOLUTE WILL DEPRESS THE FREEZING POINT AND ELEVATE THE BOILING POINT OF ANY (aq) SOLUTION COMPARED TO THE PURE SOLVENT. IN THIS QUESTION THE SOLVENT IS NaCl, WHICH WILL CAUSE THE SOLUTION TO HAVE A HIGHER BOILING POINT AND LOWER FREEZING POINT RELATIVE TO PURE WATER. THE ANSWER IS 4. 14) THE TEMPERATURE IS THE AVERAGE KINETIC ENERGY CHOICE 1 15) KINETIC MOLECULAR THEORY…DESCRIBES AN IDEAL GAS, STATES: A) IDEAL GASES MOVE IN RANDOM STRAIGHT LINE MOTION. B) ARE NOT ATTRACTED TO EACH OTHER. C) THE GAS PARTILCES DO NOT DISPLACE VOLUME (AN IDEAL GAS SAMPLE IS FUNCTIONALLY EMPTY SPACE.) D) GAS PARTICLES HAVE COLLISIONS. a)ELASTIC…WITH OTHER GAS PARTICLES NO TEMP CHANGE. b) INELASTIC…ENERGY IS GAIND OR AS THE GAS PARTICLES COLLIDE WITH CONTAINER WALL. AN IDEAL GAS WILL SPREAD (EFFUSE) TO FILL THE CONTAINER AND ASSUME ITS SHAPE, SO CHOICE 4 IS BEST.. REAL GASES DISPLAY THE KMT BEST AT HIGH T, LOW P AND WHEN THEY HAVE LOW MOLAR MASS. 16) SOME FUNDIMANTAL EQ FACTS TO KNOW: A) AT EQUILIBRIUM THE RATES ARE EQUAL FOR THE FORWARD AND REVERSE REACTIONS. B) THE CONCENTRATIONS OF REACTANT AND PRODUCTS ARE STABLE AND CONSTANT AT EQ. THEY ARE NOT EQUAL. THEREFORE: CHOICE THREE IS BEST. 17) BY DEFINITION THE HEAT OF REACTION IS THE DIFFERENCE BETWEEN PRODUCTS AND REACTANTS. CHOICE 3 ENDO (END POT) EXO 18) BY DEFINITION A CATALYST DECREASES THE ACTIVATION ENERGY, ACTIVATED COMPLEX ENERGY CONTENT AND INCREASES RATE . #2 19) BY DEFINITION A CARBON FORMS CHAINS, RINGS AND COVALENT NETWORKS 20) ALKANES ARE SATURATED, ALKENES AND ALKYNES ARE UNSATURATED (HAVE MULTIPLE BONDS). THE ANSWER IS #2. AT LEAST ONE MULTIPLE (DOUBLE OR TRIPLE) ALKANE (SATURATED) ALKENE (UNSATURATED) ALKYNE (UNSATUR) CH2CHCH2CH3 CH3CH2CCH CNH2N+2 CNH2N CNH2N-2 C(4)H2(4)+2 C(4)H2(4) C(4)H2(4)-2 C4H10 C4H8 C4H6 21) TABLE R INDICATES FUNCTIONAL GROUPS: THE STRUCTURE GIVEN MATCHES THAT OF A CARBOXYLIC (organic) ACID, CHOICE #1-AN ACID 23) BY DEFINITION OF AN ISOMER WILL HAVE: A) THE SAME MOLECULAR FORMULA. B) DIFFERENT STRUCTURES. A TERMINA DOUBLE BONDEDOXYGEN, AN ALDEHYDE, TABLE R THIS ALDEHYDE IS BUTANAL. MOLECULAR (TRUE, NOT SIMPLIFIED) FORMULA IS: C4H8O THIS KETONE IS BUTANONE. SAME MOLECULAR FORMULA DIFFERENT STRUCTURE. A MID CHAIN DOUBLE BONDED OXYGEN, AN ALDEHYDE, TABLE R C4H8O 24) THE NONSPONTANEOUS ELECTROLYTIC CELL CONVERTS ELECTRICAL (KINETIC) TO CHEMICAL (POTENTIAL) ENERGY. # 2 25) ORGANIC ACIDS ARE ONE OF THE FEW ORGANIC ELECTROLYTES, THE FUNCTIONAL GROUP –COOH RELEASES HYDROGEN IONS. #3 26) A BASE (METAL HYDROXIDE WITH GROUP ONE AND TWO METALS) IT HAS OHIONS. #1 27) BY DEFINITION, A TITRATION IS A REACTION BETWEEN 2 SOLUTIONS, ONE OF KNOWN CONCENTRATION (MOLARITY) AND ONE OF UNKNOWN CONCENTRATION. #4 28) BY DEFINITION: AN ACID IS A HYDROGEN ION (NAKED PROTON) DONOR, H+ #2 29) IN ALL NUCLEAR REACTIONS INCLUDING FISSION, MATTER IS CONVERTED TO ENERGY. THE LOSS IS CALLED MASS DEFECIT. #3 30) RADIOACTIVE DECAY OCCURS WHEN UNSTABLE NUCLEI DECAY TO RELEASE RADIATION, I-131 RELEASES BETA PARTICLES ACCORDING TO TABLE N. #4 31) AS YOU READ LEFT TO RIGHT IN PERIOD 3 YOU ARE FILLING THE THIRD SHELL, THE VALENCE SHELL. AS THE NON METALS ARE ON THE RIGHT THEY HAVE MORE ELECTRONS AND THE METALS HAVE LESS. #1 32) BY DEFINITION METALS ARE: a) SOLID STATE CONDUCTORS WITH “SEA OF MOBILE ELECTRONS” b) LOW IONIZATION ENERGY AND ELECTRONEGATIVITY, AND THEREFORE EASILY OXIDIZED. c) MALLIABLE, LUSTROUS AND DUCTABLE. d) FOUND TO THE LEFT OF THE METALLOIDS. #3 IS THE ANSWER TIN, SILVER. 33) THE IONIZATION ENERGY MEASURES THE TENDENCY FOR AN ATOM TO LOSE THE MOST LOSELY HELD ELECTRON. IT DECREASES AS THE GROUP IS READ TOP TO BOTTOM. THE ANSWER IS RUBIDIUM # 3, WHICH IS BOTTOM MOST IN THE GROUP. 34) IN IONIC COMPOUNDS THE OXIDATION STATE OF THE METAL IS INDICATED BY A ROMAN NUMERAL IN THE FORMULA, THEREFORE THE IRON MUST BE +3 AS BELOW IN IRON(III)SULFIDE. #2. EACH S is 2IN BINARY COMPOUNDS. Fe HAS MULTIPLE OXIDATION STATES AND HAS NO RULE, SOLVE FOR IT AS THE VARIABLE X Fe2S3 0 THE CHARGE OF THIS IONIC COMPOUND IS 0, ALL THE SUM OF THE OXIDATION STATES MUST EQUAL 0. NO CHARGE WAS GIVEN IN QUESTION, ASSUME NEUTRAL 2(3) + 3(-2) = 0 X = OXIDATION STATE OF Fe = 3+ 35) BY DEFINITION THE PERCENT MASS IS MASS OF THE PART /MASS OF THE WHOLE * 100. FOR A COMPOUND IT IS MASS OF THE ELEMENT OVER THE MOLAR MASS * 100. % MASS COMP. = 1 X S * 100. MgSO4 % MASS COMP. = 1 X 32 g = 27% 120 g/mol 36) GASES BECOME LESS SOLUBLE AS TEMP INCREASES. LOOK ON TABLE “G” FOR THE NEGATIVE SLOPE, THE GAS HCl IS THE ANSWER THEREFORE, #1. 37) ACCORDING TO THE LAW OF CONSERVATION OF MASS (IN A CHEMICAL REACTION) , THE MASS OF THE REACTANTS (10.0 (H2)+ 80.0 (O2)) MUST EQUAL THE MASS OF THE PRODUCTS. HERE IT IS 90, CHOICE 2 39) NOTICE THE Zn IS ELEMENTAL (0) AS A REACTANT AND POSITIVE AS A PRODUCE. H2 IS ELEMENTAL AS A PRODUCT AND POSITIVE AS A REACTANT…THIS IS A HINT OF REDOX. REMEMBER SINGLE REPLACEMENT IS REDOX, CHOICE #3 Zn + H2SO4 ZnSO4 + H2 0 1, 6, -2 2, 6,-2 0 38) FORMULA B CAN BE SIMPLIFIED TO LOWER TERMS OF SUBSCRIPTS, C2H6 SIMPLIFIES TO CH3. THEREFORE FORMULA A IS THE EMPIRICAL (SIMPLE) FORMULA AND B IS MOLECULAR (TRUE) FORMULA. CHOICE 3. 40) HEAT MOVES FROM THE WARMEST TO COOLEST OBJECT, HERE THE CYLINDER (HEAT SOURCE) WARMS THE WATER(HEAT SINK), THEN THE WATER (HEAT SOURCE NOW) WARMS THE AIR (HEAT SINK). CHOICE 4 41) THE 2 SYMBOLS SHOULD BE INDEPENDENT AND MIXED AFTER THE PROCESS, NO NEW GROUPINGS (MOLECULAES) HAVE FORMED. CHOICE 1 42) THREE AND FOUR ARE THE IS THE ONLY CHOICE WHERE THE CONCENTRATION ONLY IS CHANGED, THE SAMPLES ARE BOTH STILL LUMPY, ONLY ONE FACTOR AT A TIME CAN VARY. CHOICE 4 43) AT CONSTANT PRESSURE TEMPERATURE AND VOLUME ARE IN A DIRECT PROPORTION, IF T DOUBLES, V DOUBLES…CHARLES LAW. 200 TO 400 IS A DOUBLING, #2. 44) THE HEATING OF WATER WITH A CHANGE IN TEMP IS CALCULATED BY: q = MC∆T OR GET ∆T ; 1200= 36(4.18)∆T q = MC(T2 –T1) ∆ T = 8, THUS THE TEMP REACHES 30 C 1200 = 36g (4.18) ( T2 – 22) = 30 C 45) Br2 HAS AN IONIC CHARACTER OF 0, THERFORE ITS BONDS ARE NON POLAR AND THE MOLECULE IS NON POLAR . IODINE IS ALSO NON POLAR. A)NONPOLAR MOLECULES HAVE VAN DER WAALS WEAK INTERMOLECULAR ATTRACTIONS B) VAN DER WAALS BECOME STRONGER AS MASS INCREASES, IODINE IS MUCH HEAVIER THAN BROMINE., THUS IODINE HAS THE STRONGER INTERMOLECULAR FORCE, CHOICE 4 46) IN NUMBER THREE, AN ELEMENTAL METAL AND ELEMENTAL NON METAL GO FROM 0 (ELEMENTAL) TO AN OXIDATION STATE: CHOICE 3 Fe + S FeS 0 0 +2, -2 47) BY DEFINITION AN ACID REACTS WITH A BASE TO GIVE SALT AND WATER. LiOH IS A METAL HYDROXIDE, A BASE. THEREFORE LOOK FOR THE ACID IN AN ANSWER CHOICE. #4, H2SO4 YOU CAN ALSO USE TABLE K AND L TO FIND ACID/BASE. 48) THE WORD NEUTRALIZE INDICATES A TITRATION: MAVA = MBVB (1.0) (24) = (2.0) (X) 12 Ml = X VOL OF BASE NEEDED. 49) NUCLEAR FUSION IS THE MOST EXOTHERMIC PROCESS KNOWN, AND IT IS A NUCLEAR REACTION. THE OTHER THHREE ARE CHEMICAL REACTIONS AND EVOLVE FAR LESS ENERGY. CHOICE 3. 50) NATURAL TRANSMUTATION HAS ONLY ONE REACTANT, THERE WILL BE NO BOMBARDMENT PARTICLE. CHOICE 4