SBI 4U1 Strand B: Biochemistry Review
advertisement

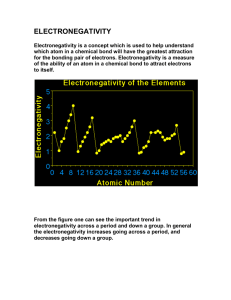
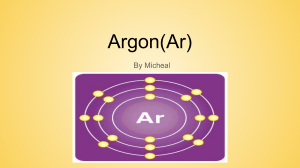
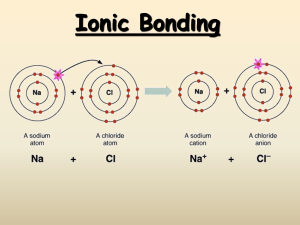
SBI 4U1 Strand B: Biochemistry Review 1. Determine the difference between an element and a compound. 2. Describe the structure of an atom. (Use a diagram to support your answer) 3. If an atom has a Atomic number of 15, determine the number of protons, neutrons and electrons it will have. Show your calculations and draw a Bohr-Rutherford diagram to support your answer. 4. What is an Isotope? How are isotopes tracers used in the medical field. 5. What feature do the following elements have in common? a. Lithium b. Sodium c. Potassium d. Rubidium 6. How many electrons are found in the outer energy shells of the following elements? a. Bomine b. Argon c. Phosphorus d. Barium 7. What characteristics are shared by the following elements? a. Helium b. Neon c. Argon d. Krypton 8. What factor determines the chemical behaviour of an element. 9. Explain what is meant by the term Valence. 10. Explain the importance of the Electronegativity of an element. 11. For each of the following paired elements determine which one has the greater electronegativity. a. K and Na b. O and Li 12. What is an Ion? How is it created? Explain how Ionic bonds are formed. 13. What is a Covalent bond? How is it different from an Ionic bond? 14. Draw an electron dot diagram for the following molecules and compounds. a. O2 b. C2H6 c. NH3 d. CO2 e. H2O 15. Write the structural formula for each of the above. 16. Why is a water molecule polar? 17. What are the three intermolecular bonds? Explain. 18. Explain the difference between Hydrophilic and Hydrophobic molecules. 19. Which of the following elements would you expect to react with the element chlorine: a. Hydrogen b. Neon c. Sodium d. Carbon 20. Write the chemical formula for each successful reaction in question 19. Revised Sept. 2010