Electronegativity & Bond Polarity Worksheet
advertisement

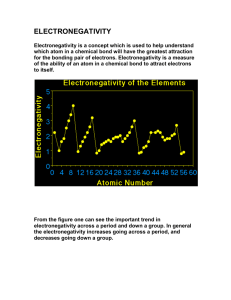
Name:___________________________________________ Period _______ Date _______________________ Section 6.1 Practice Worksheet Using Electronegativity to Predict Polarity of Bonds Part 1 The property of ______________________ , which is the measure of an atom’s ability to attract electrons, can be used to predict the degree to which the bonding between atoms of two elements is ionic or covalent. The _______________ the electronegativity difference, the more ______________ the bonding is. If the calculated electronegative difference is… > 1.7 : ionic bond is formed > 0.3 , < 1.7 : polar-covalent bond 0 – 0.3 : non-polar covalent bond Directions: Fill in the following chart, using the electronegativies of the elements in each suggested pair. Elements Electronegativity Element 1 Element 2 Electronegativity Difference Bond Type Mg to Cl H to O C to Cl N to H C to S K to F Na to Cl H to H Part 2 Looking at the types of bonds you identified above, do you notice any trend for each pair between the location on the periodic table of each element and their calculated electronegativity difference? Metal & nonmetal : __________________ Nonmetal & nonmetal : ___________________ It is also possible if a compound contains polyatomic ions, for both types of bonding to be present. Ex: Name:___________________________________________ Period _______ Date _______________________ Directions: Classify the following compounds as ionic, covalent, or both. 1. CaCl2 ____________________ 5. BaSO4 ____________________ 2. CO2 ____________________ 6. H2O ____________________ 3. MgO ____________________ 7. SO3 ____________________ 4. HCl ____________________ 8. AlPO4 ____________________