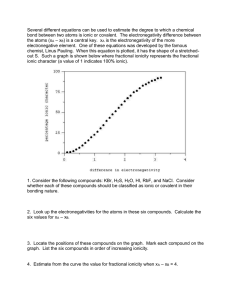
Identifying and Naming Compounds
advertisement
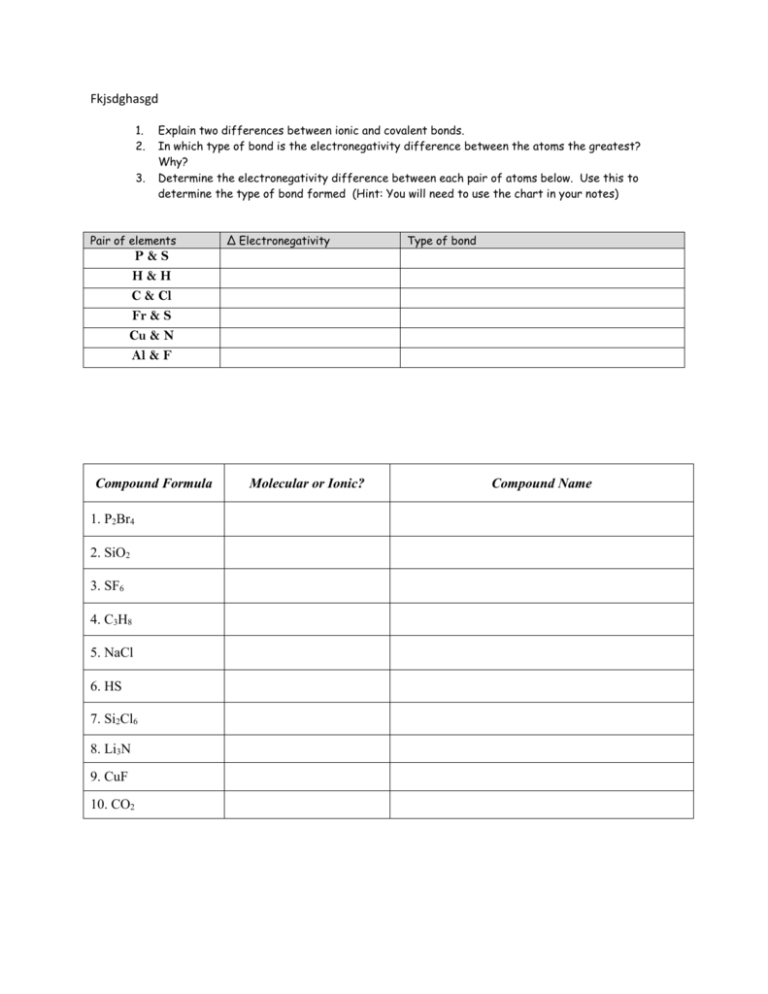
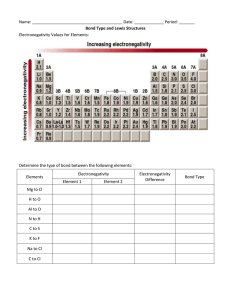
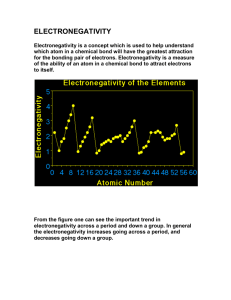
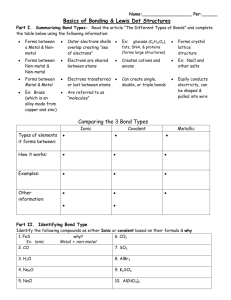
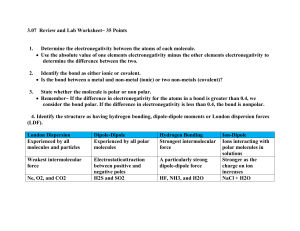
Fkjsdghasgd 1. 2. 3. Explain two differences between ionic and covalent bonds. In which type of bond is the electronegativity difference between the atoms the greatest? Why? Determine the electronegativity difference between each pair of atoms below. Use this to determine the type of bond formed (Hint: You will need to use the chart in your notes) Pair of elements ∆ Electronegativity Type of bond P&S H&H C & Cl Fr & S Cu & N Al & F Compound Formula 1. P2Br4 2. SiO2 3. SF6 4. C3H8 5. NaCl 6. HS 7. Si2Cl6 8. Li3N 9. CuF 10. CO2 Molecular or Ionic? Compound Name