Clinical Research Assistant (IM-Reno)
advertisement

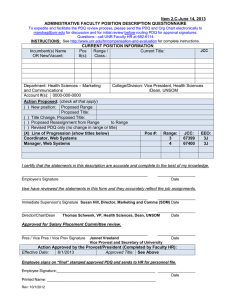
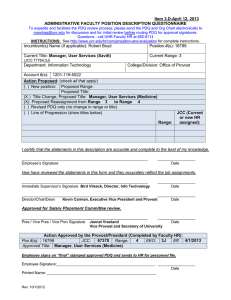
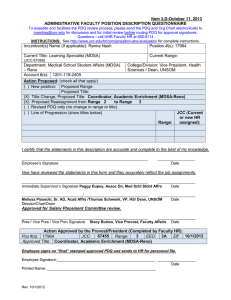
Item 9.B-August 5, 2011 ADMINISTRATIVE FACULTY POSITION DESCRIPTION QUESTIONNAIRE To expedite and facilitate the PDQ review process, please send the PDQ and Org Chart electronically to marshag@unr.edu for discussion and for initial review before routing PDQ for approval signatures. Questions - call UNR Faculty HR at 682-6114 INSTRUCTIONS: See http://www.unr.edu/vpaf/hr/compensation/placement.html for complete instructions. Incumbent(s) Name (if applicable): Joann Oches; New position Position #(s): 16974; new Current Title: Clinical Research Assistant (IM-Reno) Current Range: 2 (JCC:76993;3H;CM9505;CC330;E) Department: Internal Medicine - Reno College/Division: University of Nevada School of Medicine (UNSOM/MDDN) Account #(s): 1320-119-08IV; -001K Action Proposed: (check all that apply) ( ) New position: Proposed Range: Proposed Title: ( ) Title Change, Proposed Title: ( ) Proposed Reassignment from Range to Range (X) Revised PDQ only (no change in range or title) (add 2nd position number0 ( ) Line of Progression (show titles below) Range: JCC (Current or new HR assigned): I certify that the statements in this description are accurate and complete to the best of my knowledge. ____________________________________________________________ Employee’s Signature __________________ Date I/we have reviewed the statements in this form and they accurately reflect the job assignments. ____________________________________________________________ Immediate Supervisor’s Signature Evan Klass Chair, Internal Medicine - Reno ____________________________________________________________ Director/Chair/Dean Thomas Schwenk Vice president, Health Sciences / Dean, UNSOM __________________ Date __________________ Date Approved for Salary Placement Committee review. ____________________________________________________________ __________________ Pres / Vice Pres / Vice Prov Signature Jannet Vreeland Date Vice Provost and Secretary of University Action Approved by the President (Completed by Faculty HR): Position #: 16974 EEO Code: 3H CUPA Code: CM9505 Exempt: Yes or No Census Code: 330 Job Class Code: 76993 Range: 2 Effective Date: 8/1/2011 Approved Title: CLINICAL RESEARCH ASSISTANT (IM-RENO) ____________________________________________________________ __________________ Employee Signature Date (Employee signs and sends to HR for personnel file after PDQ has been “final” stamped for approval) Rev: 12/1/2008 Position Description – Clinical Research Assistant (IM-Reno) Page 2 1. Summary Statement: State the major function(s) of the position and its role in the university. Attach an organizational chart with positions, ranges, and names for the division which reflects the position in it as well as those supervised in the department. (This section is used for advertisement of the position.) Under the supervision of the Associate Professor, Internal Medicine and Nutrition, who is the physician for the patient, the Clinical Research Assistant coordinates the care of patients who are part of a research protocol. The incumbent participates in the assessment, evaluation and retreatment of patients enrolled in research protocols in the Internal Medicine Department at the University of Nevada Reno School of Medicine (UNSOM) through the Practice Plan. The Assistant coordinates Internal Medicine protocols and educates medical staff who will be administering the investigational drugs. As necessary, the Assistant provides education to patients, families, and other members of the health team. 2. List the major responsibilities, including percentage of time devoted to each. Provide enough detail to enable a person outside the department to understand the job (percentage first with heading and then bulleted information). 70% - Research and Data Management Conducts all research activities within an ethically based framework and in accordance with good clinical practices, i.e., Federal, State, and Institutional regulatory requirements Assures subject safety Develops and/or evaluates new research protocols considering feasibility of conduct at the site Maintains ongoing communication with Institutional Review board (IRB) throughout all phases of the trial Participates in informed consent process Prepares site for study initiation Coordinates ongoing conduct of study through to completion Coordinates all activities for study close out Facilitates timely completion of Clinical Trial Agreement (CTA) or Grant proposal Maintains test article accountability Manages financial aspects of the clinical trial Collaborates with other departments to plan and implement relevant aspects of the protocol Maintains study records according to Sponsor and/or regulatory requirements; documents and manages data 20% - Budget Management Effectively participates as a team in budgetary guidelines and projects Manages financial aspects of the clinical trial including quarterly and annual updates as required Oversees clinical research billing accounts payable, accounts receivable, and provide flow sheets for active and closed clinical trials Provides contract negotiation budgetary analysis for clinical projects, updating as needed 10% - Compliance and Professionalism Actively supports the Department and University in accordance with University policies and maintains competency Commits to efficient, effective quality care and service for patients, families, visitors, physician and team members Communicates directly and honestly and uses complete and open communication in an appropriate manner and in an appropriate setting Position Description – Clinical Research Assistant (IM-Reno) Page 3 Provides potential solutions and/or ideas when accompanied by criticism and problem identification Participates in the development of the facility. Uses facility resources efficiently. 3. Describe the level of freedom to take action and make decisions with or without supervision and how the results of the work performed impact the department, division and/or the university as a whole. Level of Freedom: The individual works closely with the Chair of Internal Medicine on decisions associated with the position, but must work independently, with initiative, and with some latitude in decision-making. Impact: The decisions and judgments made by the position affect the quality of oncology research performed, its compliance with the requirements of the protocol, and the ability of the Internal Medicine Department to continue to participate in such research. Accurate and complete performance of the job duties associated with this position is crucial to patient health. Failure to perform as expected could result in substandard patient care. 4. Describe the knowledge, skills (to include cognitive requirement and verbal and written communication), and abilities (to include task complexity, problem solving, creativity and innovation) essential to successful performance of this job (in bullet format). Knowledge of: Requirements of each protocol and ability to ask providers for assistance Determination, performance and/or scheduling of protocol required test and procedures Application of regulatory requirements including International Conference on Harmonisation (ICH), Health Insurance Portability and Accountability Act (HIPPA), Occupational Safety and Health Administration (OSHA), International Air Transport Association (IATA) Federal Guidelines for use of animals and human subjects in research, University IRB, electronic records Coding requirements Medical records and consent laws Medical terminology Skills: Screen patients for eligibility for designated protocol Strong verbal and written communication skills including syntax, grammar, and spelling Working knowledge of computers, equipment and software used to perform duties including Microsoft Word (including creating/editing tables, headers/footers, outlines, and other formatting), Microsoft Outlook and Excel (including data entry, ability to create and edit spreadsheets) Appropriate phone and customer service skills Ability to: Adequately make judgments and decisions Meet unchangeable and uncontrollable deadlines Have a very high degree of attention to detail Multi-task, organize, and prioritize Work both as a team member and independently Position Description – Clinical Research Assistant (IM-Reno) Page 4 Perform other essential duties and responsibilities efficiently, accurately and safely Use ICD-9 and CPT4 coding books 5. Describe the type of personal contacts encountered in performing the duties of the job. Explain the nature and purpose of these contacts: i.e., to provide services, to resolve problems, to negotiate. Internal UNSOM faculty, residents and staff IRB and contracting research organizations and their representatives Practice Plan employees; MEDSchool Associates North employees University of Nevada School of Medicine employees Reason for Contact To communicate with in order to fulfill requirements of protocol External Clinical research organizations and representatives Government Agencies Insurance Companies Physicians, other health care providers Patients, families, visitors Reason for Contact To communicate with to fulfill the organization’s requirements Vendors, suppliers To provide services and to communicate with in order to fulfill requirements To work alongside in same setting with some shared patients; to work with staff to recruit patients; correspondence; trials conducted on premises To prepare common administrative paperwork; to work directly with PIs who are on the study that are also UNSOM faculty To communicate with in order to fulfill regulatory requirements To communicate relating to shared patients, as required To discuss shared patients; education and coordination of activities relating to regulatory or other requirements To provide instruction and assistance in participation for study; ongoing education To contract negotiation; discuss billing or payables 6. Indicate the minimum qualifications which are necessary in filling this position should it become vacant. Please keep in mind the duties/responsibilities of the position rather than the qualifications of the incumbent. a. Minimum educational level, including appropriate field, if any. Bachelor’s Degree from a regionally accredited institution in a science related field b. Minimum type and amount of work experience, in addition to the above required education necessary for a person entering this position. Bachelor’s Degree and four years or a Master’s Degree and two years of experience with: IRB training or familiarity of IRB process Clinical or administrative experience in clinical trials EKG experience Preferred Licenses or Certifications: None Position Description – Clinical Research Assistant (IM-Reno) c. Indicate any license or certificate required for this position. Current Certificate in Pulmonary Resuscitation (CPR) Collaborative Institutional Training Initiative (CITI) Certificate (dealing with human subjects protocols) International Air Transport Association (IATA) Certificate (shipment of dangerous/biological products) Page 5