1. Please propose a mechanism for the conversion of 1 to... the relative stereochemistry of 3.
advertisement
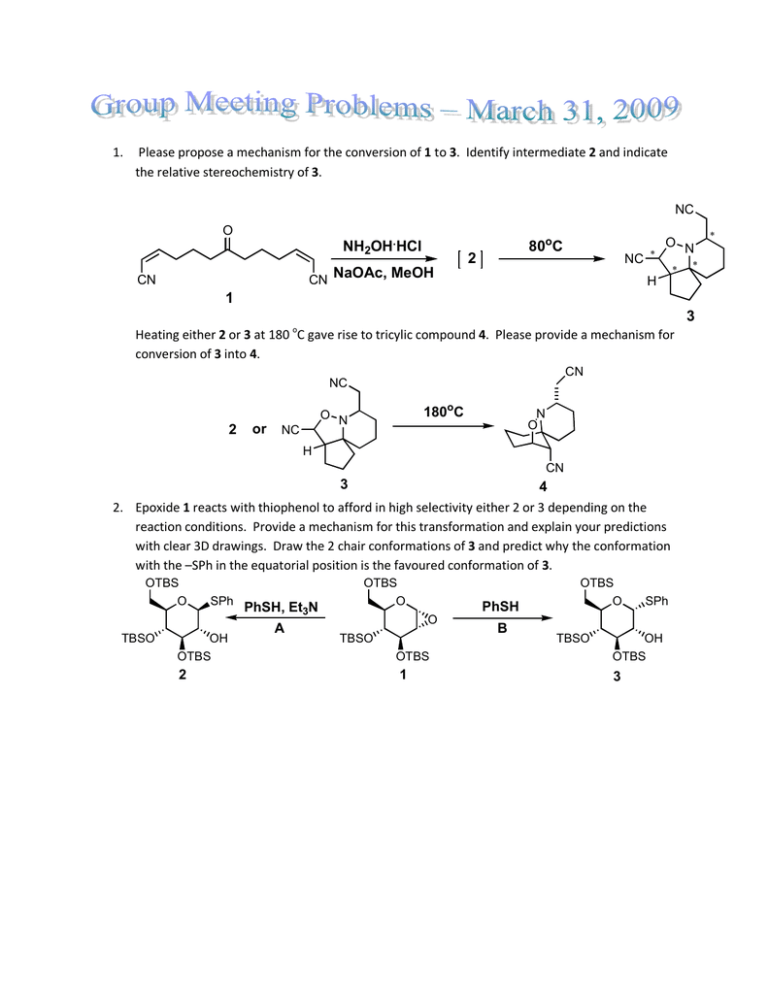
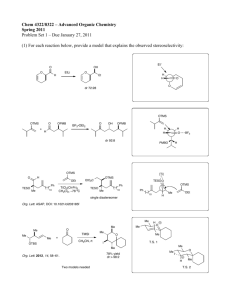
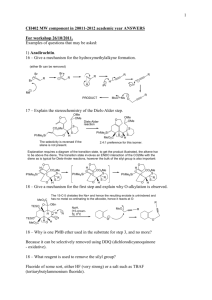
1. Please propose a mechanism for the conversion of 1 to 3. Identify intermediate 2 and indicate the relative stereochemistry of 3. NC O NH2OH.HCl 80oC 2 ∗ NC ∗ CN NaOAc, MeOH CN O N ∗ H 1 3 Heating either 2 or 3 at 180 oC gave rise to tricylic compound 4. Please provide a mechanism for conversion of 3 into 4. CN NC 2 or 180oC O N NC N O H CN 3 4 2. Epoxide 1 reacts with thiophenol to afford in high selectivity either 2 or 3 depending on the reaction conditions. Provide a mechanism for this transformation and explain your predictions with clear 3D drawings. Draw the 2 chair conformations of 3 and predict why the conformation with the –SPh in the equatorial position is the favoured conformation of 3. OTBS O TBSO OTBS SPh OH PhSH, Et3N A OTBS O O PhSH O TBSO B TBSO SPh OH OTBS OTBS OTBS 2 1 3 ∗