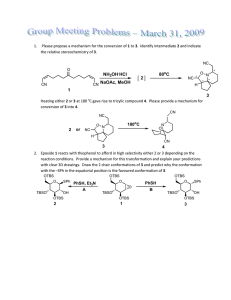
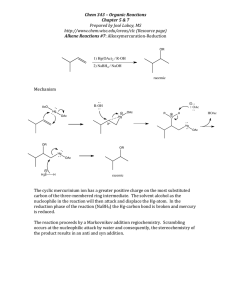
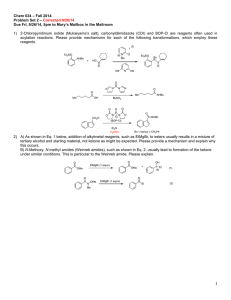
Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Introduction Nathan Wilde January 2014
advertisement

Nathan Wilde Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Introduction January 2014 The elements: Tellurium: Discovered in 1782 as a gold telluride mineral. Named from tellus, the Latin word for "earth." The percent of relative elemental abundance for the universe is far higher than that on Earth, partly because Te forms TeH2, which is volatile so it escapes the Earth. Te has no biological function, but some fungi can incorporate it into peptides in the place of S or Se. Thallium: Discovered in 1861. It produced a green spectral line by flame spectroscopy, so it is named after thallos, the Greek word for "a green shoot or twig." It is usually at the +1 or +3 oxidation state. Tl+ ions are similar to Ag+ and K+ in size, and in vivo they are pumped into cells through potassium channels, and once inside the cell thallium binds to sulfur in cystein residues and ferrodoxins. Tl3+ ions resemble softer versions of boron and aluminum Lewis acids, and are also a potent oxidants. Lead: Discovered roughly 9000 years ago in the middle east. In atomic physics, 208Pb is "double magic" because it has 82 protons and 126 neutrons, making it excpetionally stable to radioactive decay. In animals, lead accumulates in the tissues and bones, as well as attacks the nervous system. Why are they not used? Tellurium: Not very toxic, but relatively rare and expensive. Humans exposed to as little as 0.01 mg/m3 or less of Te metal in air exude a foul garlic-like odor known as 'tellurium breath'" (Wikipedia: "Tellurium"). Most organisms metabolize tellurium to dimethyl telluride, the source of the smell. Other organo tellurides do not smell bad. Mostly perception is why it's not used. Thallium: Crazy toxic. "Poisoner's poison": Tl(I) salts are tasteless and odor-free. Also, symptoms of thallium poisoning are similar to other illnesses, so physicians are often confused. Unlike mercury and lead, however, thallium is not a bioaccumulative poison. Tellurium Lead: Toxic, but not as toxic as public perception leads you to believe. By weight, palladium is more toxic than lead, and some authors in the literature claim palladium is ten times more toxic. Lead is abundant and has many industrial applications, so you're more likely to be affected by it. Pb tends to have high ligand coordination numbers (4-7, even as high as 8 or 9), so making as using well-defined organoplumbanes can be difficult. Relative abundance in the earth's crust Element ppm Oral LD50 for rat (Sigma Aldrich's MSDS's) Lead 14 Pb(OAc)2: 4665 mg/kg 2.2 Tin Pd(OAc)2: 2100 mg/kg Thallium 0.6 TlCl: 24 mg/kg Iodine 0.14 TeO2: > 5000 mg/kg Note: Diarrhea Tellurium 0.005 Platinum 0.003 SeO2: 68.1 mg/kg Gold 0.0011 Lead Thallium Main Sources: "Main Group Metals in Organic Synthesis", 2004, edited by H. Yamamoto and K. Oshima. Chapters 9, 13, and 15. Reviews: Te: Synthesis 1991, 793 & 897. Tetrahedron 2005, 1613. Chem. Rev. 2006, 1032. Tl: Synthesis 2010, 1059. Synthesis 1999, 2001. Acc. Chem. Res. 1970, 338. Pb: Tetrahedron 2001, 5683. Tellurium reagents and making C-Te bonds Al2Te3 + 3H2O See book chapter Homogeneous production of Ti(III) species in inert solvents 3H2Te + Al2O3 Ph Na/NH3 Na2Te (or Na2Te2) NaBH4/EtOH $1/g (Strem) RM + Te RTeM Na2Te RX Na2Te2 R = alkyl I O2 I (RTe)2 R2Te ArX + Na2Te Ar2Te X = I, or N2BF4 (requires heat) (RTe)2 Ph H Ph H D2Te (from Al2Te3 + D2O) $5/g (Aldrich) Cl OH Ph Works on aliphatic aldehydes and ketones too, but with lower yields. OD Ph D D2O is a cheap way to reductively deauterate things. 100% O Ph H CO2Et Other reactions with similar mechanisms: Reformatsky-type, epoxides with an -LG give allylic alcohols, dealkylation of quaternary ammonium salts, removal of nitro groups, removal of sulfones, and more. See the above review. JOC 1992, 6598 Telluronium Ylides ... do the same things as sulfonium ylides ACIE 1980, 1009 H2Te (from Al2Te3 + H2O) CO2Et TeCl4 + RTeX3 100% O (PhTe)2 NaBH4 53% Reduction O Bull. Chem. Soc. Jpn. 1986, 3013 TePh RTeR' TeCl3 (RTe)2 + X2 OH Only diasteromer. With aqueous TiCl3 they get a mixture of this and the meso isomer. 99% Dehalogenation R'X Ph Ph DCM, rt H Chem. Lett. 1986, 1339 NaTeH M = Li, Na, MgX OH iBu2Te, TiCl4 O $25/g (Materion) Te Tellurium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde H2Te (from Al2Te3 + H2O) O Ph 89% H Other reductions possible with Te reagents (such as NaTeH, PhTeSiMe3): aryl alkenes, enemines and imines, nitrones, thio carbonyls, nitro groups, N-oxides, azides. See the reviews, especially Synthesis 1991, 793. LiTMP Te(iBu)2 O Te(iBu)2 THF, -78°C TMS , -unsaturated esters and ketones can also undergo cyclopropanation. Ar H TMS O Ar TMS H Tet. Lett. 1983, 2599 If your HWE isn't working, telluronium ylides can do that too. R CO2Et KOtBu Br CO2Et then RR'CO Te(nBu)2 CO2Et Te(nBu)2 R' Note: stabilized sulfonium ylides such as these are inert to carbonyl groups. Or even a Julia-olefination-type dimerization JOC 1984, 3559 Ar Ar nBuLi SO2Ph then cat. Te TeLi Ar Halotelluration of alkynes R Ar Ar SO2Ph Te SO2Ph -LiO2SPh TeCl4 Ar Li Br SO2Ph Hydrotelluration of alkynes R H M = Al, B, Zr M R Ar Ar EtOH Br Br R TeBr2Ar So what do you do with all these fancy tellurides you can make? Metal-tellurium exchange and direct cross-coupling! R BuLi Ph EtOH Te Bu Ph 75% R R3 Te Bu 77% OTBS TenBu 72% This method also works great for Michael-addition on alkynes bearing an EWG. You can even trap with an electrophile stereospecifically, opening possibilities for stereodefined tetrasubstituted olefins. R R TeR' Pd(0), R2M HO Ph AlEt3 R3 R Ph OTBS Me2CuCNLi2 Pd(0), n Ph CuCNLi2 88% n TenBu Ph OH R Li TeR' (nBuTe)2, NaBH4 Ph TeAr TeR' R Ph R ‡ EtO H H M Br TeBr2Ar NaBH4 anti-addition ‡ TeBr2Ar ‡ ArBr2 Te ‡ Br R R anti-addition R Ar H (R'Te)2, NaBH4 syn-addition R MeOH syn-addition R see Chem. Rev. 2006, 1032 M H R TeBr2Ar benzene Br ArTeBr3 Te -Te CHCl3, reflux Ar ArTeBr3 Li -LiO2SPh Ar Tellurium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde R2 M = SnR3, ZnR, Cu, B(OH)2 ZnEt2 R ZnEt AlEt2 Syntheses using telluride chemistry Romo's gymnodimine synthesis Me OPMB Me H SnBu3 O H Me O H O HO HN OTBS ( )-gymnodimine I O + O OH M OTBS OTIPS Me H OH O N Me Marino's macrolactin A synthesis. JACS 2002, 1664 H Me H O Tellurium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde Me CO2H Me Me Me HO ( )-macrolactin A Me OTBS O O O Me PhO2S Me Me O TeBu nBuLi, (BuTe)2 Me TBSO O Me NaBH4 Me >19:1 Z:E Me O O S Cl OEt TBSO O pTol Cl O S pTol ZnCl2, DIBAL Me MeN Me OMe TBSO Me OH O Cl S O CsF OH O S pTol pTol epoxide OTBS NaHMDS Et2AlCl, Me O O TsN OTBS TsN TBSOTf TBSO (BuTe)2 Me They got the same diastereomer using either olefin geometry, Me suggesting a stepwise DA. Me NaBH4 Barbier-type macrocyclization R N Me H O O H NHK O coupling Vinylogous Mukaiyama aldol coupling O O M OTBS TeBu [Cu] OTBS OTBS M Me OTBS BuCuCN(2-Th)Li2 Me Me ACIE 2009, 7402. Org. Lett. 2005, 5127. Org. Lett. 2000, 763 O 1. Me2C(OMe)2 2. TFAA 3. Ph3PCHCHO epoxide Me HO HO O O S pTol Me Me O O Not covered Miscellaneous telluride applications from Li-Te exchange Acyl anions JACS 1990, 455 O O nBuLi R TeBu Li tBu Me tBu Many more ways to make C-Te bonds! Allylic oxidations Telluroxide eliminations Tellurolactonization Not much on tellurium heterocycles O O R R HO Me R = Ph, 85% Acyl stannanes or selenoesters don't do this. Thallium Butenolide synthesis R Tetrahedron 2012, 10601 R 1. BuTeLi TeBu Me O OH 2. NaBH4 nBuLi, then CO2, then H3O+ O BuTe TeBu Standard Hg(II) Pd(II) Tl(III) Cr2O72Pb(IV) MnO4- O R R O Me O HO Me Tellurophene synthesis Me Tetrahedron 1997, 4199 nBuLi BuTe Li Synthesis 1999, 2001 E+ Bases in Suzuki coupling Li Li TBSO Org. Lett. 2013, 5122 Me Me O Et3B, O2 O TePh OAc Ph O O H O Ph O H Me H Me HO O AcO AcO HO Me O Ph OAc OBz trigohownin A OTBS OTBS OAc OTBS OTBS O Pd(PPh3)4 (0.25 eq) base TBSO TBSO O OTBS THF/H2O OAc OTBS (HO)2B OTBS OMe single isomer Me Me OTBS OTBS OH OAc DCM 87% No reaction with the selenium acetal. OH TBSO I E Te Radical-polar crossover reaction electrode potentials Hg(0) = +0.91 V Pd(0) = +0.915 V Tl(I) = +1.25 V 2Cr(III)= +1.33 V Pb(II) = +1.69 V Mn(II) = +1.70 V Li Te nBuLi O O Tellurium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde Ph O B O O Et O H Et O ‡ Base Time KOH 2 hr TlOH <<30 s TlOEt 30 min Ag2O 5 min JACS 1987, 4756. Yield 86% Kishi's palytoxin 92% substrates 74% 92% See also Org. Lett. 2000, 2691. O OTBS OMe These Tl(I) salts also seems to be very capable of alkylating and acylating 1,3-dicarbonyls and phenols. See Acc. Chem. Res. 1970, 338. Tl(III) Oxidative Rearrangements R OH Tl(NO3)3 R Ar -NO3 R Ar (O3N)2Tl Me Me MeOH -[O] of ketones is also possible by this mechanism, but I won't MeO show any examples. O MeO R Ar R Tl(NO3)2 OMe O 1. H2, Pd/C 2. KOH, MeOH 3. MeLi Me Me Me O CO2Me 1. PhI(OAc)2, KOH 2. HO2CCF3 76% Ring contraction MeO2C Me H O H Me Me O Me Me Ph3P=CH2 O O H H ( )-bakkenolide A Me Tl(NO3)3•3H2O O H CO2H DCM, rt, 24 hr Me To shift or to eliminate? H 90% O OH Me (III) Tl Me Tl(III) Me Me Me Me O O -Tl(I) Me OH O Ph O O Tl(OTs)3 OH Ph Tl(OAc)2 AcOH, reflux O H Ph O TsOH, reflux Tl(III) OH CO2H Ph OH Me H Tl(OAc)3 O H2O Me J. Chem. Soc., Perkin Trans. 1 1992, 2565 O Me Me Me Me Me O JOC 1998, 1716 Me 1. HMDS, TMSI 2. MeLi, then NCCO2Me O Me HC(OMe)3 MeOH Me 59% single diastereomer Tl(NO3)3 Et Iodine(III) reagents gave a 1:1 mixture of diastereomers and 40% overall yield HC(OMe)3/ MeO2C MeOH (7:3) O Synth. Commun. 1995, 3931 O Me Me Tl(NO3)3 OH Ar -TlNO3 -NO3 -aryl esters from aryl ketones OMe O JOC 2010, 2877 An example in synthesis of ( )-bakkenolide A OH O Ar Thallium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde Ph Tl(OTs)2 O Aryl shift doesn't work with electron-poor arenes. O Ring expansion Tet. Lett. 1996, 3865 Olefins and alkynes react with Tl(III) with and without rearrangement, much like other pi-acids. Here is a one-pot synthesis of coumarins. O TMSO HO Tl(O2CCF3)3 82% Ph MeCN Ph CO2H O O Tl(OAc)3 Ph O O Tl(O2CCF3)3 74% MeCN Ph TMSO HO CO2H OH CO2H (AcO)2Tl OH O Ph Tl(O2CCF3)3 H O P R cis:trans = 9:1 70% MeCN J. Chem. Res. 1998, 392 OH Phenol oxidation. JOC 1994, 5439 Estrone semisynthesis Me H H MeO AcO Br PhI(O2CCF3) does not activate the olefin, but it does do the other oxidation. Evans: JACS 1997, 3419 and refs. Completed Vancomycin w/o Tl(III): ACIE 1998, 2700 OH Zn Tl(NO3)3 O AcO AcO R O O I Tl(NO3)3 R I Tl(NO3)2 HOAr R OAr R OMe R Me O 3 steps AcO R OAr 80% H H HO estrone H R Yamamura: Tetrahedron Lett. 1996, 8791 and refs. CrCl2 one-pot MeOH OH OH AcO O Ar OH Vancomycin syntheses H2O -CH2O -TlNO3 -NO3 MeO OMe H Ar O Tl(NO3)3 MeOH HO Br O Pb(OAc)4 HOBr AcO O JOC 1995, 6499 OH Me O Me O P R Tl(OAc)2 Tl(OAc)2 O Ph HO OH polyphosphoric acid Ph TMSO AcO Thallium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde R OMe Thallium January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde Aromatic thallation OMe CHO Tl(O2CCF3)2 CHO Tl(O2CCF3)3 SnBu3 Cl HO2CCF3 N H Lead OMe CHO Pd(PPh3)4 Cl N H Cl N H Synlett 1996, 609 Making lead reagents and making C-Pb bonds For R = vinyl or alkynyl, M = Hg, Sn. For R = aryl, M = Si, Zn, B(OH)2 are Pb(OAc)4 + RM RPb(OAc)3 also used. Note: transfer with B(OH)2 requires Hg(OAc)2 as catalyst. ArH + PbX4 ArPbX3 + HX X = OAc, O2CCF3 Ar must be electron-rich Thallation then halogenation Tl(O2CCF3)3 Ar H Tet. Lett. 1969, 2427 Arylation and vinylation of enolates KI Ar HO2CCF3 (or MeCN) Tl(O2CCF3)2 Ar I CO2Bn H2O O BocHN Yield 96% 70% 98% 75% 96% 98% OH + BocHN OBn DCM, rt O O Tl(O2CCF3)3 CO2Bn NHTr Na Substrate Product benzene iodobenzene fluorobenzene o:p = 11:89 o-xylene 4-iodo-o-xylene anisole o:p = 17:83 benzoic acid ortho only 2-methylthiophene 2-methyl-5-iodothiophene Synlett 1996, 609. Tetrahedron 2001, 5683. OBn O MeO Pb(OAc)3 MeO Br O O 40% unoptimized single diastereomer Towards diazonamide. JACS 1984, 5274 Thallation then Pd-coupling O OH Tl(O2CCF3)2 styrene PdCl2 Enantioselective arylation of phenols O OLi O MeCN 80% Ph + Me Me R Pb(OAc)3 N H MeO MeO N H O brucine toluene -20°C R = iPr R = Ph H O H Me Br Yamamoto: JACS 1999, 8943 OH brucine Or you can do it with Ru, Cu, and alkynes: Org. Lett. 2012, 930. Not covered One-electron aryl-aryl coupling Triorganothallium and tetraorganothallate Reductions with Tl0 NHTr O R Me Me R 99%, >99% de, 61% ee 68%, >99% de, 83% ee Me Ph O Pb L L N* Attempts to make the same C-C bonds, much less enantioselectively, gave lower yields for Pd-catalysis and no triaryl for Ni-catalysis. Enolate vinylation towards CP-263,114 nHex O O Pb(OAc)4 CO2Me O H R (AcO)3Pb nHex O H H CO2H (+)-CP-263,114 SnMe3 O O OAc R = CH2CH2OTBDPS H SnMe3 Shair did finish the molecule. Although they didn't use the organolead vinylation, they did use the same oxyCope strategy. JACS 2000, 7424. Bioorg. Med. Chem. 2001, 347 OMe Me More functionalization through radical intermediates Carbonylation of saturated alcohols O H R Pb(OAc)4, CO benzene OH R JACS 1998, 8692 Me Me O R O 63% OMe Me Me Me Pb(OAc)4, Cu(OAc)2, quinoline CO2H H Me Me 9% Pb(OAc)4, CaCO3 H H O O Me 5 H O SnMe3 2 O HO O O Me Tet. Lett. 2000, 9655 H O BF3•OEt2 64% O O HO O 1) LiSnMe 3 2) BrMg 51% H Oxidative cleavage of C-C bonds R Bu3Sn OMe OO Shair: JACS 1998, 10784 O nHex O Lead January 2014 Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead Nathan Wilde H Me Me [O] O H R O R OH CO R O OH [O] R Tet. Lett. 1966, 1017 OH O -oxidation of carbonyls N Me CO2Bn O O O O AcO toluene, reflux N Me via: O H2, Pd/C Pb(OAc)4 O CO2Bn N CO2Bn Me O O O (OAc)2 Pb Me h O Tet. Lett. 1998, 5693 O Pb(OAc)4 O CO2H CO2H basketene! O + Na2CO3 Nathan Wilde Unused Elements in Organic Synthesis: Thallium, Tellurium, Lead It should be pointed out that more effective structural design of lead species would be possible if one could control the number of coordination sites and complex ligand exchange. Carboxylate ligands are labile and rapidly undergo intermolecular exchange. In connection with this undesirable equilibrium, concomitant formation of oligomeric or polymeric structures as a result of complex intermolecular interactions imposes significant limitations on further development in this area of research. -Taichi Kano and Susumu Saito, 2004 Not covered Pb(II) as a Lewis acid. N-arylation (lead version of a Buchwald reaction) Olefin aziridination Carbon radicals from organolead species Alkylation of aldehydes with tetraorganolead species Allylic and benzylic acetoxylation Pb(0) reductions Lead January 2014