CH402 MW component in 20011-2012 academic year * indicative
advertisement

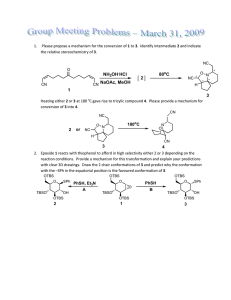
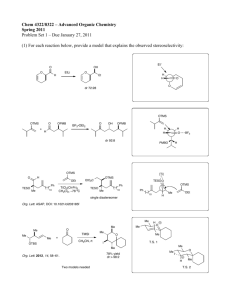
1 CH402 MW component in 20011-2012 academic year ANSWERS For workshop 26/10/2011. Examples of questions that may be asked: 1) Azadirachtin. 16 – Give a mechanism for the hydroxymethylalkyne formation. (either Br can be removed) Br Br Br : R R R R R H H O iPr Me R PRODUCT MsO Ms O 17 – Explain the stereochemistry of the Diels-Alder step. OMe OMe O S CO2Me PhMe2Si O OMe OMe O Diels-Alder reaction S PhMe2Si S H MeO2C The selectivity is reversed if the silane is not present. H OH S 2.4:1 preference for this isomer. Explanation requires a diagram of the transition state, to get the product illustrated, the alkene has to be above the diene, The transition state involves an ENDO interaction of the CO2Me with the diene as is typical for Diels-Ander reactions, however the bulk of the silyl group is also important: O O H PhMe2Si CO2Me S R S O H H PhMe2Si O CO2Me S R S O H H PhMe2Si CO2Me S R S O H 18 – Give a mechanism for the first step and explain why O-alkylation is observed. The 15-C-5 chelates the Na+ and hence the resulting enolate is unhindered and has no metal co-ordinating to the alkoxide, hence it reacts at O: R OBn O TESO NaH, MsO R [15-crowno H H H 5], 0 C TESO O O O H O H MeO C MeO2C 2 18 – Why is one PMB ether used in the substrate for step 3, and no more? Because it can be selectively removed using DDQ (dichlorodicyanoquinone - oxidative). 18 – What reagent is used to remove the silyl group? Fluoride of some sort, either HF (very strong) or a salt such as TBAF (tertiarybutylammonium fluoride). 2 18 – Provide a mechanism for the rearrangement step. It is not essential to draw the entire molecule, however enough should be drawn to indicate the correct reagent: R OPMB OBn . O H O H H O O H 18 – Provide a mechanism for the radical cyclisation: N AIBN= N CN -N2 (Bu3n)Sn . (x 2) O . R H O CN (Bu3n)Sn . Sn(nBu3) . S OBn OMe R . H O H . OBn OMe OBn OMe R O O O . Sn(nBu (tin radical goes on to initiate a further cycle . CN NC MeS H O OBn OMe 3) R O O Problem question example; ‘why is the formation of a direct bond between the two halves of the target molecule difficult to achieve? Answer would be – the two halves are very complex and highly functionalised, and leave little room for addition of functional groups to assist their connection ina reaction. 2) Strychnine. 25 – Give a mechanism for the key step. This is essentially from the notes: N HN usual condensation H HO NR2 OtBu A H HO NR2 OtBu N N HO NR2 H OtBu O NR2 H OtBu B 26 - What is the mechanism of the step? 3 EtS EtS N EtS N EtS H O NH2 N H H OSEM OPMB B H C OSEM OPMB H H N O H H N OH H N 27- Give a mechanism for the key step and explain how the stereochemistry is controlled. What other protecting groups could you use? be sure to show how one part of the molecule is positionally related to the other in the t.s. On then, you could use a carbamate such as a Boc group. N N KOtBu THF, 0.02M 80oC N H N H H O O N N N H H H H H N H N H H O O O tBu H OtBu N H H O 28 – key step A-> C mechanism. O Ag Br O NBn NBn AgOTf N H A N H CO2Me O NBn H B CO2Me NBn DBU (base) N CO2Me Show deprotonation next to ester then O protonation at O NBn gamma position as above. N H H N H H CO2Me C CO2Me Problem question example ‘A-> C why is this an efficient way to make the tetracyclic system?’ 4 An answer here would be that it makes two rings, one after the other, in an efficient process. Compare this with the previous route – indicate which is shorter, how convenient are the reagents etc. advantages, diadvantages. 3) Epothilones. 26 – provide a mechanism for the key Grubbs metathesis step. Grubbs metathesis catalyst S HO S HO N N O O TBSO O O (1.2:1 E:Z) Key Step (3:2 with other trans isomer) O TBSO O 37 – provide a mechanism for the DCC/DMAP step, and the metathesis step. DCC/DMAP: (show deprotonation with DMAP first) S HO HO O O . O TBSO O TBSO O NMe2 NCy Get H+ from NCy protonated DMAP O NCy N NCy TBSO O TBSO (complete by deprotonation) ROH N NCy O O O NMe2 N NHCy TBSO O Metathesis: You can abbreviate the Grubbs catalyst as Ru=CH2. You can also abbreviate the structure as well as long as you define the abbreviation. CH2 Ru S S HO HO N N O O O TBSO (1.2:1 E:Z) Key Step O TBSO O O CH2 Ru Ru Ru S HO N S HO N O O TBSO O O O TBSO O Problem question example ‘why is formation of a triple bond by metathesis sometimes advantageous over formation of a double bond?’. Because the double bond can form as an E/Z mixture. The triple bond can be reduced to a Z bond selectively. 5 38 – Provide a mechanism for the aldol step. S TBSO TBSO N N O O H TPSO O TMS O N TPSO O O S TMS TBSO N O Product shown after protonation. TPSO OH O Problem question example ‘why is the protecting group changed in the sequence?’. 39 – Yamaguchi lactonisation mechanism. O S S HO HO N O TBSO O First the acid is deprotonated by a mild base such as Et3N Cl O O Cl N O OTBS O Cl Following loss of TBS by adddition of Cl- O TBSO O O Cl TBS Cl S S N HO HO N OTBS OTBS Cl O O TBSO O O O TBSO Cl O Cl O Cl Cl O Cl For workshop 18/11/2011. 4) Amphotericin B (6 slides, 4 key steps). 44/45 – give mechanisms of Wadsworth-Horner-Emmons reactions in one of these two steps. 6 This is a mechanism you should already know. The first step is a deprotonation which you should illustrte (even though I have not). The anion is delocalised, although I have shown the C-based form. Partial structures are acceptable as long as you make it clear which compounds they refer to. OMe OMe OTBS O O O OTBS O O O MeO MeO OTBS O O CO2Me CO2Me O P MeO MeO O OMe OTBS P O OMe OTBS O O OTBS O MeO MeO CO2Me O OTBS O O O OTBS O O OMe OTBS O CO2Me OTBS O O O MeO MeO P O CO2Me P O 45 - Problem question example ‘What could go wrong in the macrocyclisation step’. It could do an intermolecular reaction, or polymerise, or not cyclise at all. 46 – cycloaddition mechanism. (But)O H Cl Cl TBSO N OH TBSO N O H BnO O O O O O O But O TBSO TBSO XN N N O O O O O O HO MeO O O HO MeO XN O O 47 – provide a mechanism for the key step – complete what’s shown below – this is basically from the slide and has been completed now. 7 Cl R enantiomer Ph2 P 10 mol% O of catalyst. >99% ee with a 30:1 preference for this isomer over the meso. OMe OMe Ir O Ph2P OH OH Cl OH O2 N OH Cl OAc Cs2CO3, 110oC H H Ir Ir - OAc (abbreviated catalyst) Ir OAc OAc H H H Ir O OH O Ir H H OH OH Ir O OH + H Ir H OAc 48 – Krische strategy – why take this approach, i.e. what are the advantages? It provides asymmetric and regiocontrol, you can build a chain in both directions in same step. 5) Taxol (5 slides, 6 key steps). 52 – Give a mechanism for the cycloaddition. Explain how the stereochemistry is controlled. O OH + O CO2Et O PhB(OH)2 90oC O EtO2C (Diels-Alder) O OH BPh O O O O O O CO2Et B Ph (Endo TS) O O O CO2Et B Ph 53 – Shapiro mechanism? ‘Why are lithiated alkenes difficult to form?’ Because alkene protons are not very acidic. Other positions tend to deprotonate first. The mechanism is shown below. 8 TBSO O TBSO H i) nBuLi (shapiro reaction) OTBS OBn OTBS ii) OBn O O H O HO H N NSO2Ar nBu O H nBu TBSO After protonation, which you should show in the answer OTBSOBn TBSO TBSO O H O N N SO2Ar O N N HO O OH O OBn O H O O O OBn TiCl3.DME Zn-Cu McMurry Coupling O H O O O O O TiCl3 + Zn/Cu -> Ti(II) (or Ti(0) O Ti(II) O O Upon work up and hydrolysis. If you use Ti(0) it will be oxidised to Ti(IV) Ti(IV) O OBn OBn 53 – Mechanism of McMurry coupling, What are strengths of this approach? The mechanism is shown above. There is some debate as to whether it is Ti(0) or Ti(II). I will accept either. The important thing is the chelation control. It forms a C-C bond in a stereoselective manner. 55 – Why does the epoxide open, i.e. what drives the reaction? Release of a lot of ring strain drives it. 56 – mechanisms of both key steps should be known. A->B is a standard dihydroxylation which you should know. C->D is an SN2-type cyclisation.. 56 – why does the cyclisation of C-D proceed as shown. – Because it goes with inversion of configuration. 6) FR182877/abyssomicin C (5 slides, 5 key steps). 9 64 – Cyclisation step – why is a ‘transannular’ intramolecular cyclisation employed. Provide a mechanism for the reaction. Because the two reacting components are held close together by the ring. Mechanism is below, make sure you show the overlaps if you are asked to explain why the outcome is as shown. The mechanism is also shown on slide 65, for the other enantiomer – careful! TESO TESO H OTES O H OTES O HH OtBu OtBu H H OTMS O OTMS O TESO H OTES O H H OtBu H H H OTMS O 65 – Mechanism of the addition step. I PPh3 I I I TBSO PPh3 TBSO OTBS O OTBS I2, PPh3 CO32H OEt O then CsCO3 OEt Br OH O I PPh3 O OEt OTBS An acceptable alternative would be to form the allylic iodide then displace it with the anion. O Ph3P O 66 – Provide a mechanism for the reaction. O OTBS 10 O O Pri N H Pri O OMe O product formed after addition of acid in workup, which should be illustrated. OMe OTBS O O 67 – Provide a mechanism for the reaction. O O O O O O OMe O O O OMe O O O O O O OMe OMe O 68 – Give a mechanism for the reaction. Why do the addition before the metathesis, and not the other way round? The mechanism is shown below. If you tried to do the metathesis first, you would not have the advantage of an intramolecular cyclisation and you’d get homodimers as well as the required product. i) tBuLi then O HO O O OTES PMBO OH O O O First the tBuLi deprotonates to form the anion shown. 7) Statins (6 slides, 5 key steps). O OTES 11 77 – provide a mechanism for one or both reactions, explain how the stereochemistry is controlled in the cycloaddition. I have already shown a mechanism for the Wadsworth-Emmons reaction, which is the first step. The second step is a cycloaddition. See below – use appropriate overlaps to indicate stereochemical control. OBn OBn O OBn O O O + O O O P(O)(OMe)2 O NaH O THF H H OTBS O OTBS H OTBS 78 – Provide a mechanism and explain how the stereochemistry is controlled. TMSO TMSO O O H H H TMSO H Endo Diels-Alder reprotoantes O TMSO TMSO O deprtonates H O H H H H H 79 – Give a mechanism for the key step. What advantage does the reaction have over enolate formation? Mechanism is shown below. Note that each Sm(II) provides 1 electron in a reductive process so you need 2 eq. Of SmI2. 12 O O Br O O O O Sm(III) O O H O O 2 x SmI2 H (reductive coupling) HO H O H O O O H Product after protonationnote that protonation should be shown. H 80 – Give a mechanism for the reaction. It is the same mechanism as for slide 18 above, but a different substrate.