Chem 8322 S2012 - PS1
advertisement

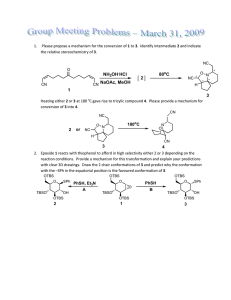
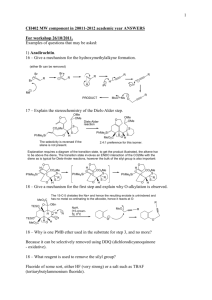
Chem 4322/8322 – Advanced Organic Chemistry Spring 2011 Problem Set 1 – Due January 27, 2011 (1) For each reaction below, provide a model that explains the observed stereoselectivity: O O Et– OH EtLi H O H H Et O O dr 72:28 OTMS OTMS O + OPMB O BF3•OEt2 OH OPMB H H H dr 92:8 [Ti] TESO Me TiCl2(Oi-Pr)2 CH2Cl2, –78 ºC 10 OTMS EtO2C OEt Ph BF3 H PMBO OTMS H O H O TESO TESO O OTMS Ph Ph 10 Me 10 H single diastereomer OEt Me Org. Lett. ASAP, DOI: 10.1021/ol203185f Me O Me TMSI Me Me Me O + OTBS Me Me CH3CN, rt H O Me T.S. 1 I Me Me 78% yield dr > 98:2 Org. Lett. 2012, 14, 58–61. Me O I– H Two models needed Me T.S. 2 [Sn] H BnO O Me O H OH SnCl4 OBn Me Me Me H [Sn] H BnO O OBn 89% yield dr > 30:1 Org. Lett. 2012, 14, 178–181. H H OTBS PMBO O O DIPEA Bu2BOTf O + Me R H H PMBO OBn PMBO O OH Bu H OTBS B O O Bu O Me Ar Me Me OBn Org. Lett. 2012, 14, 178–181. 87% yield dr 7:1 OTBS PMBO O OH Me4NBH(OAc)3 O PMBO Me O OAc Me OBn PMBO OH OH OBn Me O B H OTBS Me Ar OAc O Me OBn Org. Lett. 2012, 14, 178–181. 93% yield dr > 20:1 O OH Na NaBH4 OTBS NHBoc OTBS EtOH –78 ºC NHBoc dr 11:1 Org. Lett. 2012, 14, 382–385. BocHN O TBSO H H H BH3 Me OMe OMe BnO Et2BOMe NaBH4 O OH BnO OH H3B H Et OH THF/MeOH –78 to 0 ºC Me H R OH Me MeO 98% yield dr > 20:1 OH Me TMS3SiH AIBH O O •Si(TMS)3 Me Me O CO2Et Me single diastereomer CO2Et O BH3 OH OMOM Me 1. BH3 BzO 2. H2O2 NaOH Me H OMOM Me H H Me Si(TMS)3 (This is a radical reaction with TMS3Si• serving as the nucleophile) H O O CO2Et BzO Et O OBn H Org. Lett. 2011, 13, 5816–5819 Me B O Me Me dr 29:71 CH2OMOM Me H BzOCH2 (2) The Cornforth model is another stereoinduction model for carbonyl addition reactions that predates the Felkin-Ahn model and is based on dipole minimization. Importantly, they both favor addition to the same face of the carbonyl group (see below). A number of theoretical studies have given more support for the Felkin-Ahn model in a majority of cases. However, there are a growing number of cases for which the Cornforth model appears to be the better model. O R X H H O R H Nuc Nuc OH Felkin-Ahn model R Nuc X X O H X = OR, NR2, Cl 1,2-anti R H X Nuc Cornforth model Consider the boron-mediated aldol reactions shown below. Construct transition states for each aldol reaction using both the Felkin-Ahn and Conforth models for the aldehyde. Using these models, determine if these results are consistent with either the Felkin-Ahn model or the Cornforth model. Also explain why the aldehyde facial selectivity (ratio of 3:4) is poor in the second case (another model may need to be drawn to explain this). (Hint: consider syn-pentane interactions when building your models.) Reaction A (9-BBN)O O Me + H O OH Me 2 3 Me OTBS O Me 4 OH 2 + 4 3 Me OTBS 1 Me OTBS 2 2,3-syn : 2,3-anti = 93:07 1 : 2 = 98:02 Reaction B (c-Hex)2BO O + H Me O Me OH 2 3 Me OTBS O 4 Me OH 2 + 4 3 Me OTBS 3 Me OTBS 4 2,3-anti : 2,3-syn = 99:01 3 : 4 = 21:79 Reaction A L L B i-Pr H L mis-matched O O TBSO I O OH 2 H 3 H Me Me Me 4 Me matched L B i-Pr H O O Me OTBS H II 1 syn-pentane Felkin-Ahn model H OTBS Me Cornforth model Reaction B L L B i-Pr H matched O OH 2 Me O TBSO III L O 3 H Me H Me 4 Me mis-matched L B i-Pr H O O Me Me OTBS OTBS H Felkin-Ahn model syn-pentane H IV 3 Cornforth model O OH 2 3 Me 4 4 Me i-Pr H mis-matched O Me Me OTBS syn-pentane L B O OTBS H H V Cornforth model L In reaction A, the Felkin-Ahn model I leads to a syn-pentane interaction with the Z enolate. However, this interaction is absent with the Cornforth model II. The observed 3,4-anti selectivity is high. This points to the Cornforth being active in this case. In reaction B, the Felkin-Ahn model III would give high 3,4-anti selectivity because there is no synpentane interaction. In the Cornforth model IV there is a syn-pentane interaction that would lead to low 3,4-anti selectivity. The observed 3,4-anti selectivity is low. This points to the Cornforth being active in this case as well. The major diastereomer in reaction B (4) arises from model V for which the synpentane interaction is likely less severe than in IV.