Group Meeting 12.11.2009 - Group Renaud

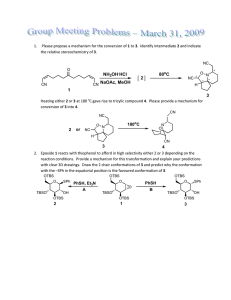
Group Meeting
12.11.2009
Total Synthesis of Vinigrol
Thomas J. Maimone, Jun Shi, Shinji Ashida, and Phil S. Baran
J. Am. Chem. Soc. Asap
10.1021/ja908194b
OH
OH
OH
• unprecedented 1,5-butanonaphtalene skeleton
• eight contiguous stereocenters
• 22 years of failures and “toward Vinigrol” papers
• several patents on the route to Vinigrol, due to its proprieties against hypertension and platelet aggregation, its abilities to inhibit the progression of ARC to AIDS and to induce necrosis in tumors
1
Corey ʼ s Biosynthetic Proposal
S. N. Goodman, Ph. D. Dissertation, Corey Group, Harvard University, 2000 .
N. Z. Burns, Baran Group Meeting, September 2007 .
2
Different Approaches Published cis-Decalin scaffold first, then octalin:
Convergent Enantioselective
Synthesis of Vinigrol , an
Architecturally Novel
Diterpenoid with Potent
Platelet
Aggregation Inhibitory and
Antihypertensive Properties.
1.
Application of Anionic
Sigmatropy to Construction of the Octalin
Substructure
Chains are nowhere near each other and they really do not want to cyclise
Paquette et alii , J. Org. Chem.
, 2003 , 68 , 6096. 3
Having Learnt the Lesson Not
Paquette et alii , J. Org. Chem.
, 2004
Paquette et alii , J. Org. Chem.
, 2004
Paquette et alii , J. Org. Chem.
, 2004
4
Selection of Other Approaches
Hanna et alii , J. Org. Chem.
, 1993
Scaffold completed, no functionalisation
Barriault et alii , Org. Lett.
, 2007
Scaffold completed no functionalisation possible
Hanna et alii , J. Org. Chem.
, 1997
Scaffold completed, dead end
Congested structure allow very few manipulations
5
Beginning of the Synthesis
OTBS
MeO
2
C
+
TBSO
2 equiv.
1) DIBAL
2) DMP
H
O
80%
H
OTBS
AlCl
3
-78 °C
MeO
2
C
OTBS
O H
65% d.r. 2:1
MgCl
1) LDA
Tf
2
2) Pd(PPh
3
LiCl
OMgCl
H
OTBS
H
O
SnBu
3
)
4
MeO
2
C
78%
H
OTBS
OH
81%
H
H
OTBS
1) DMP
O
H
1) LDA, MeI
OH
H
OTBS
3) Me
2) TBAF
4
NBH(OAc)
3
O H
92%
H
H
72%
T. J. Maimone, A.-F. Voica, P. S. Baran, Angew. Chem. Int. Ed.
, 2008 , 47 , 3054.
T. J. Maimone, A.-F. Voica, P. S. Baran, Angew. Chem.
, 2008 , 120 , 3097.
O Ms
H
OH
H
6
Grob Fragmentation
O Ms
H
OH
H
KHMDS
0 °C -> r.t.
OMs
H
O
H
TMS
N TMS
H
OH
H
OH
H
O
H
Antiperiplanar relationship
7
Dipolar Cycloaddition
O
Shielding from the proximal cyclohexone
& neobutyl prevent the reaction
O
Br
Br
OH
N
KHCO
3
Br
H
N
O
D. M. Vyas, Y. Chiang, T. W. Doyle, Tetrahedron Lett.
, 1984 , 25 , 487.
I. De Paolini, Gazz. Chim. Ital.
, 1930 , 60 , 700.
Br
N
O
H single diastereomer single regioisomer single positional isomer
88%
8
Functionalisation of the Tricycle
Br
N
O
O
H
DIBAL
O
S
S
10 equiv. NaH
20 equiv. CS
2
40 equiv. MeI
Br
N
O
H
88%
180°C
OH
Br
N
O
H
95%
Br
Crabtree
B(Oi Pr)
3
H
2
N
O
H
96%
Br
LiAlH
4
OH
N
O
H
H
2
N
OH
H
9
Saegusa Deamination
H
2
N
OH
H
HCO
2
H
MeO
N
Cl
N
N OMe H
O
N
H
OH
H
COCl
2
Et
3
N
CN
OH
H
3 equiv. AIBN
9.8 equiv. Bu
3
SnH
OH
H Me
OH
H
56% (4 steps) gram-scale
Seagusa et alii , J. Am Chem. Soc.
, 1968 , 90 , 4182. 10
Completion of the Synthesis of
(±)-Vinigrol
Me
OH
H n -BuLi
OsO
4
OH
OH TEMPO
NMO
Me
OH
H
95% n. b.: the other sec.
hydroxy group is shielded by the third cycle
NaOCl
2Li +
O
Li
(CH
2
O) n
Me
O
H
Me
OH
OH
O
TrisNHNH
2
H
OH
N
NHTris
Me
OH
H
OH
OH
51%
OH
11
As a Thought:
Redox Economy in Organic Synthesis
N. Z. Burns, P. S. Baran, R. W. Hoffmann, Angew. Chem. Int. Ed. 2009 , 48 , 2854 12