Chemistry 634: Advanced Organic Chemistry – Synthesis and Reactivity
advertisement
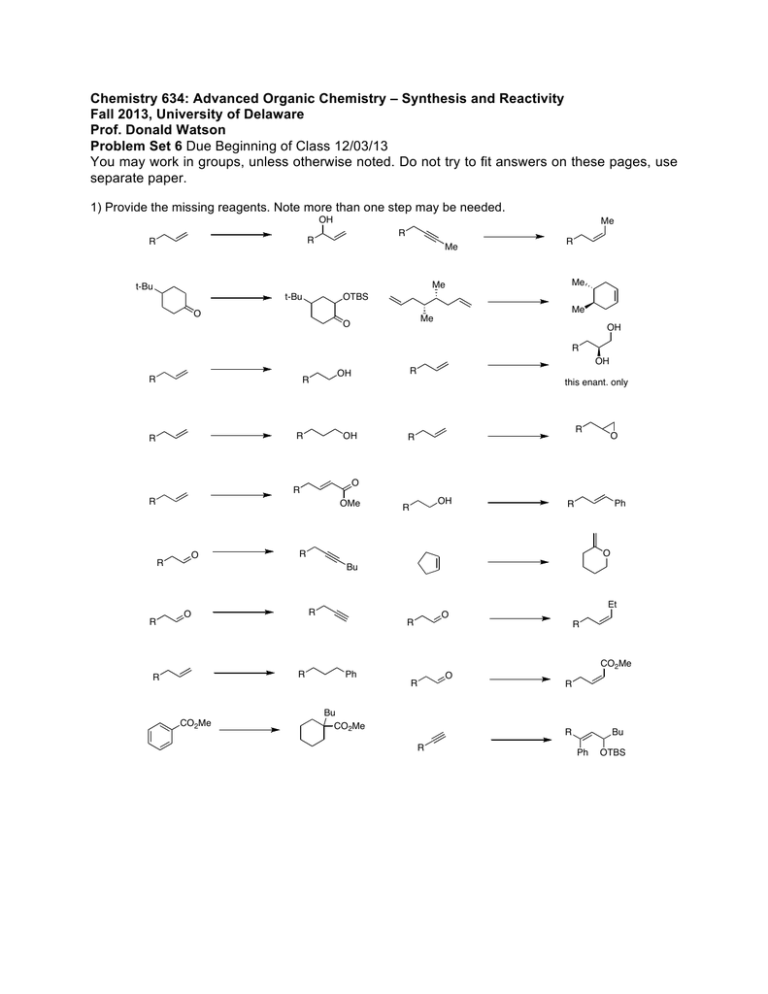
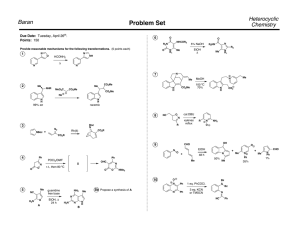
Chemistry 634: Advanced Organic Chemistry – Synthesis and Reactivity Fall 2013, University of Delaware Prof. Donald Watson Problem Set 6 Due Beginning of Class 12/03/13 You may work in groups, unless otherwise noted. Do not try to fit answers on these pages, use separate paper. 1) Provide the missing reagents. Note more than one step may be needed. OH Me R R R Me R Me Me t-Bu t-Bu OTBS Me O Me O OH R OH R R this enant. only OH R R R OH R OMe O R O O R R R R OH R Ph R O R Bu CO2Me O R R R Et R O Ph R CO2Me R Bu CO2Me O R R R Bu Ph OTBS 2) a) In class, we have discussed that Lewis acids can promote the formation of oxo-carbenium ions and trigger the addition of allyl silanes to them. Sometimes the oxo-carbenium ion can form from a suitably protected alcohol and an aldehyde. Given that major hint, rationalize the following transformation including the stereochemical outcome (DO NOT LOOK UP THE ANSWER TO THIS). O OTMS Me Me BnO CO2Me SiMe2Ph H BnO TMSOTf -50 °C O CO2Me 1, dr = 10:1 b) After 1 was prepared, it was converted to 2 in three steps. What reagents could be used to carry out this sequence? 3 steps Me BnO O Me CO2Me BnO 1 O OTBS 2 3) Predict the outcome of the following transformation and illustrate model to predict stereochemistry. Me Me H Me Cy2BH, then H2O2 H H H AcO 4) Provide a synthesis of (+)-disparlure starting from materials commercially available from major chemical companies (ie Aldrich, Acros, Strem, etc). Show all steps. Your synthesis should be expected to provide highly enantioenriched product. Provide a catalog number for each starting material. You may use online or paper catalogs for this problem. DO NOT USE SCIFINDER OR BEILSTIEN. Me Me O Me (+)-disparlure 5) Provide a synthesis of the following compound from materials commercially available from major chemical companies (ie Aldrich, Acros, Strem, etc). Show all steps. Your synthesis should be expected to provide highly enantioenriched or enantiopure product. Provide a catalog number for each starting material. You may use online or paper catalogs for this problem. DO NOT USE SCIFINDER OR BEILSTIEN. Me Me O O H OMe