Carvone in Organic Synthesis: A Chiral Pool Presentation
advertisement
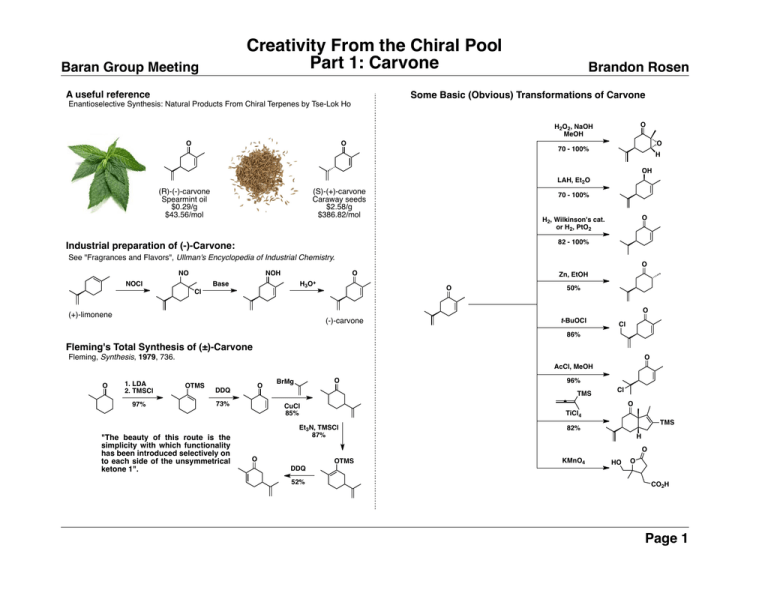
Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting A useful reference Brandon Rosen Some Basic (Obvious) Transformations of Carvone Enantioselective Synthesis: Natural Products From Chiral Terpenes by Tse-Lok Ho O H2O2, NaOH MeOH O O O 70 - 100% H OH LAH, Et2O (R)-(-)-carvone Spearmint oil $0.29/g $43.56/mol (S)-(+)-carvone Caraway seeds $2.58/g $386.82/mol 70 - 100% O H2, Wilkinson's cat. or H2, PtO2 82 - 100% Industrial preparation of (-)-Carvone: See "Fragrances and Flavors", Ullman's Encyclopedia of Industrial Chemistry. NO NOH H3O+ Base NOCl O O Zn, EtOH O Cl 50% O (+)-limonene (-)-carvone t-BuOCl Cl 86% Fleming's Total Synthesis of (±)-Carvone Fleming, Synthesis, 1979, 736. O AcCl, MeOH O 1. LDA 2. TMSCl 97% OTMS O DDQ 96% TMS 73% "The beauty of this route is the simplicity with which functionality has been introduced selectively on to each side of the unsymmetrical ketone 1". O BrMg Cl O CuCl 85% TiCl4 Et3N, TMSCl 87% TMS 82% H O O OTMS DDQ 52% KMnO4 HO O CO2H Page 1 Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting An Overwhelming Sample of Carvone-Derived Syntheses O O O O Picrotoxinin Trost, 1999 OH O N H O HO HO O O OH O H H OH H H O OH Ryanodol Deslongchamps, 1979 H O H C7H15CO2 O O N 46% O OH H OH H Omphadiol Romo, 2011 H N O O HO Platensimycin Lee, 2009 O N H Cyperolone Kirsch, Org Lett 2012, 14, 1250. OTIPS O 6 steps O OTES PtCl4, cod R OTES O 80% [M] Briarellin F Overman, 2003 Samaderine Y Shing, 2005 Br N H (N)-Methylwelwitindolinone C Isothiocyanate Trilobolide Ley, 2003 HO NaNH2 H H O OH Salsolene Oxide Paquette, 1997 OTBS H 13 steps O O OH O TBSO O HO N H Hapalindole Q H SCN O HO HO 3 steps Cl O OEt OPiv 4. NaOMe 5. vinyl-MgBr, CuBr•DMS OAc O O O CP-263,114 core Yoshimitsu, 2000 H OH Cubitene Lindel, 2012 O HO OH H O Guanacastepene E Sorensen, 2006 NCS 1. LAH 2. PivCl 3. CrO3 OH Valerianonoid A Srikrishna, 2004 BnO HO O H Garg, JACS 2011, 133, 15797. H OH O O (N)-Methylwelwitindolinone C Isothiocyanate OH O Cyrneine A Gademann, 2012 Peribysin E Danishefsk, 2008 MOMO 53% N H 7-deacetoxyalcyonin acetate Overman, 1995 CO2H 4 steps O O AcO Kainic Acid Fukuyama, 2011 AcO CHO H HO CO2H HO H H LHMDS, then [Cu] + N H OH Cyperolone Kirsch, 2012 Hapalindole Q Baran 2004 HO O O HO N H OMe Hapalindole Q Baran, JACS 2004, 126, 7450. O H NCS Brandon Rosen OH OH CO2H OTIPS O 8 steps H O Chinensiolide B O Hall, 2010 HO O O Cyperolone Page 2 Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting Brandon Rosen Kainic Acid Fukuyama, Org Lett 2011,13, 2068. O 1. H2O2, NaOH 2. H2SO4 3. NaIO4 4. I2, KI I O 1. NaClO2, NaH2PO4 O I CHO Hectogram Scale NHCO2Me Name rxns? "In view of the ease of the entire operation and the use of the readily available and inexpensive reagents, we are convinced that our synthetic route alone would satisfy the global demand for (-)Kainic Acid." Br 5 steps HO O O N CO2Me Kainic Acid O OH OH O O Corianin 7-Deacetoxyalcyonin Acetate Picrotoxinin Overman, JACS 1995, 117, 10391. R3SiO Br O H PPh2 O OSiR3 OSIR3 P P 1. t-BuLi, 9 steps H CHO TMS OMe OH OHC OTIPS O Pd(OAc)2 Ligands 70 % O 2. PPTS 3. Me6Sn2, [Pd] NIS CN 3 steps OHC I 1. H2 2. LDA, PhN(Tf)2 O CO2H BF3•OEt2 OTIPS OH Name rxn? TMS Br MeO2C 7 steps O MeO2C O O O Br 3. LiCH2CN 4. C5H5NHBr3 O O Trost, JACS 1996, 118, 233. Trost, JACS 1999, 121, 6131 and 6183. 1. LDA, CH2O 2. TBDMSCl O 4 steps CO2H N H 2. LHMDS, TBHP OH CO2t-Bu CO2H 1. Me2NCH(OMe)2 Ac2O O 3 steps Picrotoxinin and Corianin O HO H O MeO2C O HO 11 steps O 2. MeOH, ∆ O PhO P N3 OPh O MeO2C O H Br O R3SiO O R3SiO H Br H R3SiO O OSiR3 OSIR3 Name rxn? H I O 1. NiCl2-CrCl2 2. Ac2O 3. TBAF Name rxn? H HO H O AcO OH 7-deacetoxyalcyonin acetate Page 3 TMS Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting Briarellin F Brandon Rosen Guanacastepene E Overman, JACS 2003, 125, 6650. Sorensen, JACS 2006, 128, 7025. 1. 9-BBN, then NaOH, H2O2 O O 1. H2, PtO2 2. LDA, MeI O 1. LDA, TMSCl, then AcOH O 2. LAH (> 20:1 d.r.) 2. TEMPO, NCS H OH H H O C7H15CO2 OH CN LHMDS OH O 58% 2. [Pd], (Me3Sn)2 O O Briarellin F [Pd] 87% Gademann, ACIE 2012, 51, 4071 OTBS 4 steps TBSO 3 steps O O O PMP O AcO PMP O O O Name rxn? PMP 1. SmI2 O 2. PhSeBr 3. mCPBA 175 °C 81% O TBSO TBSO O O hv 82% CHO OTBS 2. EDCI O 1. NfF SnMe3 HO 1. NaCN OH O Cyrneine A O O 3. O3, then H2, Pd/C H O OHC AcO 1. PhNTf2 2. CrO3 8 steps TBSO O H O CHO PMP O 5 steps OH O H TBSO 3) [Pd] O Guanacastepene E O Cladantholide 1. CH2Br2, LiTMP 2. BuLi Lee, JACS 1997, 119, 8391. O TBSO HO OTBS 3 steps OH 1. H2O2, NaOH 2. LiCl, TFA 3) DHP, PPTS O Cl OTHP NaOMe MeO2C OTHP 95% Name rxn? O CHO Cyrneine A Page 4 Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting Brandon Rosen Upial Taschner, JACS 1985, 107, 5570. O O MeO2C 3 steps OTHP Bu3SnH THPO 99% O HO 4 steps H O H H OMe O 1. TsOH, MeOH 2. PCC 4 steps HCl 70% THPO 1. MeMgBr 2. OsO4, NaIO4 3. KOCl, MeOH H H 3 steps O OEt Name rxn? O O O OHC 4. Swern 5. SOCl2 MeO2C O H Upial H H OMe O O H 3. LDA, TMSCl 4. DMDO O H Name rxn? Br EtO 4 steps OH Name rxn? Omphadiol Romo, ACIE 2011, 50, 7537. O O O Cladantholide Thapsigargins OTHP H 12 steps H OTES O OH Grubbs OEt O OH O Thapsivillosin F PPY+ 2. TsCl, 4-PPY O OH 92% 2 steps Grubbs 95% OAc O H HO OO 1. H5IO6 OMOM TBDPSO O O 63% Ley, ACIE 2003, 42, 5996 See "Steven Ley" group meeting MeO2C [Mn(dpm)3] PhSiH3, O2 H O H O Br OMOM OH OH TBDPSO H OTES O 3 steps O H OEt H 1. tBuLi, DIBAL H O 2. Et2Zn, CH2I2 Name rxn? OH H Omphadiol Page 5 Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting Paquette, JACS 1997, 119, 2767. See "Leo A. Paquette" group meeting O HO TBSO O TBSO AcO Salsolene Oxide 1. H2, Pt 2. O3 Br 5 steps CO2H CO2Et 3. NaBH4 4. HBr, EtOH H (COCl)2 H H O HO OH 5 steps O H Brandon Rosen O O H OMe H H H O O Samaderine Y Et3N, ∆ 57% Platensimycin Lee, JACS 2009, 131, 8413. O O O 1. SeO2 1. LAH Bu3SnH O SPh O 2. NBS Br O TMSC(Li)N2 2. PCC CHO Br 81% Salsolene Oxide Samaderine Y Shing, ACIE 2005, 44, 7981. See "Traditional Chinese Medicine" group meeting O 3 steps O 1. LDA, CH2O 2. (OMe)2CMe2 O O TBSO O TBSO MgBr 1. O OAc 1. TFA 2. TBSOTf O 2. NaH, crown ether 3. Ac2O OH O O Peribysin E Danishefsky, JACS 2008, 130, 13765 O 6 steps H H 7 steps 2. Pd(OAc)2 TBSO O TBSO O OAc O OTMS 1. EtAlCl2 PhMe, 180 °C 62% TBSO O TBSO CO2H O O HO Platensimycin 3. NMO, TPAP O OH H N O O TBSO H H H H O 1. MnOAc3, TBHP OMe TBSO O TBSO AcO O 2. MnOAc3, ∆ H H H O 1. EtAlCl2 H O 3. Pd(OAc)2 OMe H O O OTES 2. SiO2 O H CHO O 3 NaBH4, CeCl3 OH O TBSO O TBSO O 65% 1. TBSOTf 2. TBHP, NaOH O 3. CrO3 4. NaBH4, CeCl3 3 steps O O Adduct not available through Robinson Annulation! Page 6 Creativity From the Chiral Pool Part 1: Carvone Baran Group Meeting Brandon Rosen Carvone-Derived Diene Ligands 1. OsO4, NaIO4 H O Carreira, JACS 2004, 126, 1628. Carreira, Org Lett 2004, 6, 3873. TMSN3 2. mCPBA AcO H O I2 ~ 1:1 d.r. OMe O 1. NBS, MeOH 2. KOtBu O TESO OTBS 3 steps AcO OTES H OTBS 89% O H TiCl4 50% HO H CHO HO 1. iBuLi 1. NBS, MeOH 2. KOtBu O 1. LDA, allylBr 2. LiNEt2, PhNTf2 3. [Pd], HCO2H OMe H Ar ~ 1:1 d.r. OMe O O HCl, MeOH OH AcO 2. PCC OTBS H 2. ArZnCl, [Pd] O I Suzuki OMe 1. LDA, PhNTf2 O 80% OH OMe ent-Peribysin E Ecklonialactones A and B Fürstner, JACS 2010, 132, 11042. O O Ph O B Ligand A O O [Rh(C2H4)2Cl]2 Ligand A 80% ee O 4 steps O Ph O MeO(Me)N O O Name rxn? MeO P MeO O N2 8 steps H O O MeO(Me)N O Ecklonialactone A (∆6,7) Ecklonialactone B Page 7 Baran Group Meeting Creativity From the Chiral Pool Part 1: Carvone Some Basic (Less Obvious) Transformations of Carvone Brandon Rosen Do it at home! Derive retrosyntheses of the following molecules using carvone as a starting material! 4 steps H HO O H OH H OH CO2H Gibberellic Acid O O 3 steps H OH OH O HO HO Dictyoxetane O Amphidicolin Cafestol I H H O HO OH O HO Cyclooctatin OH Phorbol H O 3 steps HO OH O O OMe O 4 steps MeO2C OTHP H CO2Et O HO Solanoeclepin A N O H O Br O HO O O 4 steps H O Pleuromutilin CO2H HO H O O OH H OH H O OHC H OH H H CHO HO H O 2 steps OH CO HO H I 4 steps H O O O O Limonin OH O H HO H MeO2C CO2Me O Nominine OMe H Mandapamate O 3 steps O Page 8