152/252: Synthetic Methods in Organic Chemistry Final Exams
advertisement

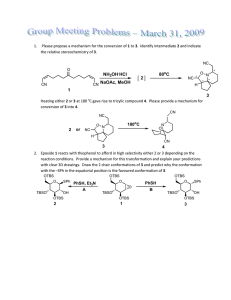
Name: Student ID number: 12/12/07 152/252: Synthetic Methods in Organic Chemistry Final Exams Instructions: 1. Please write your name at the top of every page. Please write your Student ID number at the top of the first page, right below your name. 2. The exams are from 11:30-2:00 pm. 3. This exam is composed of 8 pages total. Page 8 is blank. 4. This is an open book exam, and you are free to consult your notes anytime during the exam. However, the more time you spend looking at your notes, the less time you may have to finish the exams.... 5. Answer the questions concisely, within the provided space and erase any irrelevant structure/reagent or unnecessary information. If you need additional space please use the back side of any page. 6. Your grades, exams and related material will be available for pickup from 12/15- 12/30 from my assistant's office at PacHall 5224B. 7. The grades will be submitted to the department on 12/15/07. GOOD LUCK !!! and HAPPY HOLIDAYS!!! Problem Points Score 1. 60 ________ 2. 60 ________ 3. 60 ________ 4. 60 ________ 5. 60 ________ TOTAL 300 _______ 1 Name: PROBLEM 1: (3 parts, 60 points) 1. The Diels Alder reaction between diene A and dienophile B gives a mixture of products C and D in a ratio: C/D = 1/6. Me CO2Me CO2Me Me + Me + CO2Me A B C (10%) D (60%) a. Explain the regioselectivity of the reaction using a pneumonic device and 1-2 sentences. pneumonic device showing charges generated at transition state: Me Me CO2Me A1 Formation of D is favored because the methyl substituent of the diene stabilizes the partial charges as shown in structure A1. CO2Me D Me CO2Me Me CO2Me A2 20 points C What product ratio would you expect (approximately) for the following reactions, if performed under identical conditions as above and why (answer with 1-2 sentences). MeO CO2Me CO2Me MeO + b. MeO + CO2Me E F G H The methoxy substituent at the 2-position of the diene stabilizes better than the methyl group the partial charges formed at the DA transition state (shown as A1 above). Based on this, I expect the ratio of G/H to be smaller than that of the ratio C/D (which is 1/6). Compound H should be formed as the major product in better than 6/1 ratio. 20 points I predict that the ratio of G/H= 1/9 MeO SO2Ph SO2Ph MeO + c. MeO + SO2Ph F G F H The sulfonyl group of the dienophile is a better electron withdrawing group than the ester (structure F). Thus the DA reaction would proceed more efficiently and with higher selectivity in favor of product H. The pneumonic device A1 applies better here than in the cases above. Based on this, I predict that the ratio of G/H= 1/20 20 points 2 Name: PROBLEM 2 (60 points): a. Provide all reagents and conditions that you can use to achieve the following transformations. Multiple steps are needed. Possible sources of deuterium are: CDCl3, LiAlD4, NaBD4, NaCNBD3, Bu3SnD, D2O, D2, C6D6, CD3CO2D, Et3SiD). OH D O H A B CrO3•pyr 20 points for correct answer -5 points per wrong reaction or other oxidants BF3•Et2O Et3SH O Cl O O OH O O DIBAL-D O OH D or other reductants mCPBA (Baeyer-Villiger) D OH C CrO3 H2SO4 (Jones [O] or other [O] N O OH D 20 points for correct answer -5 points per wrong reaction S OH h! Bu3Sn-D O O N S DCC 3 Name: b. Give the structure of products or reagents A-H O G O3, then NaBH4 (excess) B E O NaIO4 OsO4 A DMSO, (COCl)2 -78 °C then Et3N NMO OH D CH3-PPh3 I 1. TBSCl, Et3N (excess) 2. mCPBA NaHMDS H2, Pd/C (excess) H C O OH OH F O O OTBS O O OH OTBS H A OH C OH B Li(tBuO)3AlH O OTBS or CH2 NaCNBH3 D H O mild reducing reagents selective for aldehydes E OH OH H CH2 O 20 points total -3 points per wrong answer OH G F H OH 4 Name: PROBLEM 3 (2 parts 60 points): Using the starting materials A and C and any reagents of your choice suggest an enantioselective synthesis of compounds B and D respectively. (Possible sources of deuterium are: LiAlD4, DIBAL-D, NaCNBD3, Bu3SnD, D2O, D2, Et3SiD). O OH enantioselective synthesis D A B – DET [O] D D 30 points total +/-10 points for enantioselective step OH NaIO4 OH OH O TBAF SAE (-) DET Ti(OiPr)4/ tBuOOH SAE gives access to one enantiomer TBSCl imid O D LiAlD4 O OTBS OH OH OTBS OH OH enantioselective synthesis Me D C CrO3 H2SO4 (Jones [O] O OH O LiAlH4 O Ph O O Me 30 points total +/-10 points for enantioselective step PhCH2O-Li+ N O Me SOCl2 Ph O H N O O O Ph Cl N Et3N Ph 5 LDA MeI O O Evans oxazolidinone forms one enantiomer Name: PROBLEM 4 (60 points) Prostanglandins are a family of extremely potent hormone-like compounds with many biological functions, including muscle stimulation, lowering of blood pressure and generation of inflammation. A synthetic scheme towards prostanglandins as developed by E. J. Corey is shown below. (Corey, E. J. et al. J. Am. Chem. Soc. 1969, 91, 5675; 1971, 93, 1490). Complete the synthetic scheme toward prostanglandin PGF3a by adding the appropriate reagents and/or providing the structure of the products. (More than one reagents/reactions may be correct for each step. Full credit will be given even if you have performed the desired transformation(s) using less/more steps than the ones indicated). NC Cl MeO 1. KOH, H2O MeO OMe Cu(BF4)2 NC 2. Cl 1. NaOH, then H+ 2. KI3, NaHCO3 6 pointd/box no partial O O O 1. O O O O BBr3 1. Ac2O, pyrid I 2. Bu3SnH, AIBN, 80 °C 2. PCC AcO AcO HO OMe OMe H O Ac: O Me O then H+ 1. Ph3P 2. O O mCPBA O THP: O K2CO3, MeOH THPO O 3. OTHP , TsOH 1. 2. HO CO2H AcOH, H2O DIBAL-H PPh3 then HO H+ CO2H HO PGF3a OH THPO 6 OTHP CO2 Name: PROBLEM 5 (60 points) Complete the synthetic schemes, by adding the appropriate reagents and/or providing the structure of the products. More than one answers may be correct. Full credit will be given if more/less steps are used than the ones indicated. H O O H 1. TBSCl, imidazole H O Ph O H H 2. 9-BBN, then H2O2, NaOH OH Ph O O H H H OH OTBS TBSCl : tBuMe2Si-Cl 1. PCC 12 points/box 2. Ph3P=CHCO2Et H O Ph O H H O O H OTBS OH O 1. DIBAL-H 2. (–)DET, Ti(iPrO)4 tBuOOH Ph O O H H OTBS CO2Et 1. PCC 2. [ Ph3P=CH2 ] 1. H O Ph O TBAF 2. PPTS, CH2Cl2 or other acids, LA H H O O O H Ph OTBS 7 O H O H H OH O