Handout - Mr. P's AP Science Site
advertisement
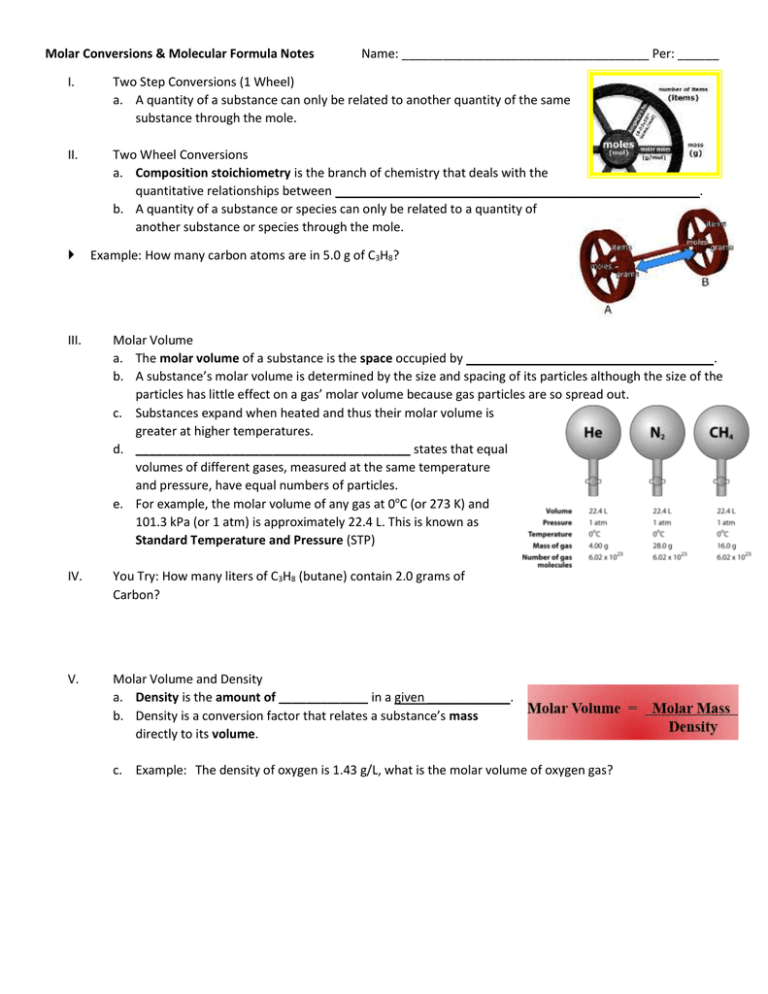
Molar Conversions & Molecular Formula Notes Name: ____________________________________ Per: ______ I. Two Step Conversions (1 Wheel) a. A quantity of a substance can only be related to another quantity of the same substance through the mole. II. Two Wheel Conversions a. Composition stoichiometry is the branch of chemistry that deals with the quantitative relationships between _____________________________________________________. b. A quantity of a substance or species can only be related to a quantity of another substance or species through the mole. Example: How many carbon atoms are in 5.0 g of C3H8? III. Molar Volume a. The molar volume of a substance is the space occupied by ____________________________________. b. A substance’s molar volume is determined by the size and spacing of its particles although the size of the particles has little effect on a gas’ molar volume because gas particles are so spread out. c. Substances expand when heated and thus their molar volume is greater at higher temperatures. d. ________________________________________ states that equal volumes of different gases, measured at the same temperature and pressure, have equal numbers of particles. e. For example, the molar volume of any gas at 0oC (or 273 K) and 101.3 kPa (or 1 atm) is approximately 22.4 L. This is known as Standard Temperature and Pressure (STP) IV. You Try: How many liters of C3H8 (butane) contain 2.0 grams of Carbon? V. Molar Volume and Density a. Density is the amount of _____________ in a given ____________. b. Density is a conversion factor that relates a substance’s mass directly to its volume. c. Example: The density of oxygen is 1.43 g/L, what is the molar volume of oxygen gas? I. Percent Composition Percent Analysis of H2O What is the percent of Hydrogen in H2O? II. What is the percent of Oxygen in H2O? Empirical Formula a. The empirical formula is the _________________________________ ratio of atoms in a compound. Percent composition in chemistry deals with masses so when calculating it, one must consider the grams that each atom contributes to the molecule or compound.) b. The molecular formula is the: ACTUAL FORMULA of a compound giving the numbers of each type of atom III. Steps to Finding Empirical Formula a. Determine the grams of each element are present (if not given numbers, use % composition data) Change % of mass to grams (assume you have 100 g) Example: 55% 55g b. Convert grams to MOLES (divide by atomic mass) (empirical formula is comparison of moles) c. Divide each answer by the smallest number of moles d. Round your ratio to the nearest WHOLE number. You can round 1.97 to 2, but you can’t round 1.333 to 1. If there is a decimal like 1.33 – multiply ALL the subscripts by 3. How about 2.5? Multiply all the subscripts by 2. Need to be able to recognize common ratios. If needed, multiply all the subscript numbers by the smallest whole number that will get them to be whole numbers Your set of whole numbers represent the subscripts for each atom in the empirical formula. Helpful Rhyme: % to mass, mass to mole, divide by small, times ’til whole. IV. Example of Finding Empirical Formula An unknown compound is composed of 81.8% carbon and 18.2% hydrogen. Determine the empirical formula for the compound. Percent to mass Mass to mole Divide by small Multiply 'til whole Practice Finding Empirical Formula An unknown compound is composed of 39.99% C, 6.71% H and 53.29% O. Determine the empirical formula. V. Molecular Formula a. Can be the same as empirical formula or a whole number ratio of it. b. Examples: H2O is the empirical & molecular formula for water. CH2O is the _________________ formula for glucose, ethanoic acid, and methanol. The molecular formula for glucose is C6H12O6, (six times the empirical ratio!) VI. Steps for Finding Molecular Formula a. Find the empirical formula b. Calculate the empirical formula mass c. Divide the molecular mass (given in problem) of the compound by the empirical formula mass d. Multiply each of the empirical formula subscripts by the whole number ratio (found in c) VII. Example of Finding Molecular Formula A compound has an empirical formula of CH2 and a molecular mass of 42 g. Determine its molecular formula. Practice Finding Molecular Formula A compound has an empirical formula of CH2 and a molecular mass of 56g. Determine its molecular formula. Additional info: Sometimes a chemical reaction gives a product that has never been obtained before. In such a case, a chemist determines what compound has been formed by determining which elements are present and how much of each is there. This data can be used to obtain the formula of the compound. Previously, we used the formula of a compound to determine the mass of each element present in a mole of the compound. To obtain the formula of an unsworn compound, we do the opposite. That is, we use the measured masses of the elements present to determine the formula. -Empirical formula can be found from the percent composition of the compound. -Molecular formula is the exact formula of the molecules present in a substance. Molar Concentration I. Molarity – A useful unit of concentration a. ___________________________ is any expression of the proportion of a chemical in a solution. b. Molarity (M) is the number of _______________ of the chemical per liter of solution, i. Ex: 1.8 M HCl means 1.8 mol HCl per litre of solution. c. The molar concentration of a chemical is indicated by putting ________________________________ around the chemical’s formula i. Ex: [HCl]. II. Preparing a standard solution a. A standard solution is a solution of ______________________________________________. b. Example of preparing a standard solution: Explain how you would make 0.25 L of a 1.5 M sodium chloride solution III. Ions in solution a. The dissociation equation provides the ___________ of the ____________________ ions to each other and to their ________________ compound. b. CaCl2(s) c. Example: How many chloride ions are in 0.125 L of a 1.5 M CaCl2 solution









