Unit 07 Percent Comp and Empirical Form
advertisement

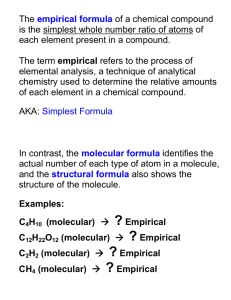
Percent Composition and Empirical Formula Percent Composition General Strategy • Convert whole number ratio (moles) to mass percent Formula Mass Percent Percent Composition Steps • Multiply each whole number subscript by the appropriate molar mass. • Divide the part of each molecule and divide by the total. • Multiply by 100%. Example 1 • What is the percentage of each element in SO2? Example 2 • What is the percentage of each element in Ca(OH)2 ? Empirical Formula General Strategy • Convert mass percents to whole number ratio (moles) Mass Percent Formula Empirical Formula Steps • Assume each given percentage is a mass • Divide each given percentage by the appropriate molar mass. • Divide all values by the smallest number of moles to obtain a whole number ratio Empirical Formula • Definition: The formula with the simplest whole number ratio. Decimals you should not just round: • 0.5 = ½ • 0.25 = ¼ • 0.33 = 1/3 • 0.66 = 2/3 Molecular Formula • Definition: A molecular formula may be simplified to an empirical formula.