Percent Composition & Formulas Chemistry Worksheet
advertisement

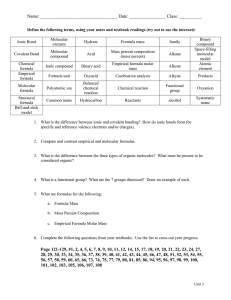
Name ________________________________ Hour ____________ Date ____________ Part I Answer the following questions about percent composition. 1. Find the percent composition of each element in the following chemicals: a. Al2(SO4)3 b. C6H8O6 2. Which of the following titanium containing minerals, rutile (TiO2) or ilmenite (FeTiO3) has the larger percentage of titanium? Show your work to prove your answer. Part II Using the following information find first the empirical formula of the compound and then the molecular formula. Circle both the empirical and molecular formulas. 3. Glycerol is a thick, sweet liquid obtained as a byproduct of the manufacture of soap. Its percent composition is 39.12 % carbon, 8.75% hydrogen, and 52.12% oxygen. The molar mass is 92.09 g/mol. What is the molecular formula for glycerol? 4. Analysis of a compound containing chlorine and lead reveals that the compound is 59.37% lead. The molar mass of the compound is 349.0 g/mol. What is the empirical and molecular formula of the compound? 5. Analysis of a chemical used in photographic developing fluid indicates a chemical composition of 65.45% carbon, 5.45% hydrogen and 29.09% oxygen. The molar mass is found to be 110 g/mol. What is the molecular formula of the compound?